Left ventricular outflow tract obstruction due to a left ventricular myxoma that was misidentified as an accessory mitral valve tissue
Introduction
Cardiac mass detected on echocardiography can be suspicious for vegetation, cardiac thrombus, cardiac tumor, such as a myxoma, or an accessory mitral valve tissue. Eighty percent of cardiac myxomas occur in the left atrium, 7% to 20% in the right atrium, and about 10% in atria, the right ventricle, or the left ventricle (LV) (1). LV myxomas are very rare, comprising only 1.7% of all cardiac myxomas (2). Particularly, it can be difficult to distinguish the LV mass when it invades the mitral valve. We report obstruction of the left ventricle outflow tract (LVOT) caused by cardiac myxoma that was misidentified as an accessory mitral valve tissue via transthoracic echocardiography (TTE) preoperatively, and intraoperative transesophageal echocardiography (TEE) attributed to make diagnosis of LV myxoma and to choose the surgical approach.
Case presentation
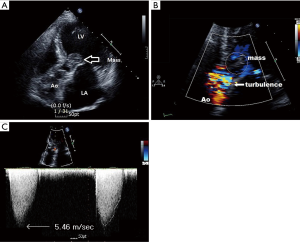
A 65-year-old woman presented with chest discomfort that persisted for 7 days. She had no other symptoms and her hemodynamics remained stable. However, TTE revealed a mobile, low-echogenic, balloon-shaped mass attached to the anterior mitral valve leaflet. The mass connected to a papillary muscle near the anterolateral papillary muscle via the chordae (Figure 1A), and was suspected to be an accessory mitral valve tissue. The mass protruded into the LVOT, leading to LVOT flow acceleration, with a peak pressure gradient of 119 mmHg (Figures 1B,C,2,3). It looked like attached to the papillary muscle (Figure 4) and the anterior mitral valve leaflet (Figure 5). Although the patient might have lived with the mass for years, she presented with chest discomfort that persisted for 7 days recently and had the history of cerebral infarction. Therefore, the surgeon considered an increased risk of sudden death and systemic embolization decided to excise the mass by emergency operation.




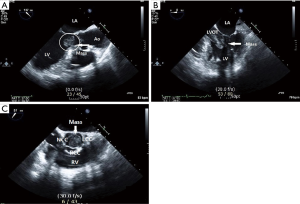
Intraoperative TEE performed under general anesthesia revealed a 2 cm × 3 cm, oval-shaped, homogeneous, and mobile LV mass. On midesophageal LV long-axis view, the mass had irregular margins and was pedunculated, with a stalk originating from the LV wall and extending to the lateral chordae of the mitral valve (Figure 6A). Based on these findings, the mass was considered to be an LV myxoma rather than an accessory mitral valve tissue. On midesophageal 5-chamber view, the mass was attached to the anterior mitral valve leaflet, appeared to originate from the mitral valve, and had a hyperlucent area in the center (Figure 6B). On midesophageal aortic valve short-axis view and midesophageal LV long-axis view, the mass protruded into the LVOT during systole, occupying the entire lumen and resulting in LVOT obstruction (Figures 6C,7). The mass was attached to the anterior leaflet, which induced mitral valve prolapse and mild mitral regurgitation.

The surgeon excised the mass partially via a transaortic approach. The LVOT was filled with the myxomatous mass, which was yellowish and gelatinous and had a stiff stalk. The mass invaded the lateral papillary muscle, lateral anterior chordae, and anterior leaflet. The mitral valve could not be preserved and was replaced with a prosthetic valve. Postoperative TEE confirmed no residual mass and normal prosthetic valve function. Histopathologic diagnosis confirmed the mass as a myxoma. The patient had an uneventful postoperative course and was discharged on postoperative day 18.
Discussion
An LVOT mass detected on echocardiography can be suspicious for vegetation, cardiac thrombus, cardiac tumor, such as a myxoma, or an accessory mitral valve tissue, rarely, but it could be particularly difficult to distinguish these diseases when the LV mass invades the mitral valve. Intraoperative TEE is the procedure of choice for the differential diagnosis. The characteristics and information of the mass (morphology, attachment site, and margin) could be collected via intraoperative TEE.
In this report, the patient showed no signs related to endocarditis, which excluded the possibility of vegetation. We first suspected an accessory mitral valve tissue because the stalk was not clearly visible on preoperative TTE, and the mass grew up into the LV with high pressure. However, on intraoperative TEE, the mass had a stalk, originated from the LV wall, and was not a general form of an accessory mitral valve tissue. Yoon et al. reported that the most important feature of a cardiac myxoma is its narrow stalk, which is useful when there is diagnostic confusion (8). In addition, a myxoma is homogeneous and may have a hyperlucent central area, indicating hemorrhage and necrosis. Calcification with echogenic foci also may be detected (1).
Although cardiac thrombi appear more frequently than cardiac myxoma (9), it usually located in the atrium. Sometimes, it has stalk and can be misdiagnose as a myxoma (10). In addition, cardiac thrombus generally occurs in patients with organic heart disease (11), or in patients with hypercoagulable disease. We also considered the possibility of the cardiac thrombus, but the patient has no history of organic heart disease and hypercoagulable disease.
Therefore, we considered that the mass was more likely to be a myxoma, which was confirmed by surgical and histopathologic findings. The initial preoperative TTE finding of a papillary muscle located near the anterolateral papillary muscle and connected to the chordae was likely a stalk rather than a muscle as initially assumed.
Patients with a cardiac myxoma are usually asymptomatic or have nonspecific symptoms, which are dependent on the location, size, mobility, and friability of the mass. This patient presented with chest discomfort although we do not know the exact mechanism for it, we postulate it is related to functional LVOT obstruction because it resolved after mass resection. However, most clinical symptoms related to LV myxoma are caused by embolization and LVOT obstruction. The high pressure of the LV during systole could increase the risk of systemic embolization, while systolic prolapse of the mass via the aortic valve could result in severe LVOT obstruction and sudden death (12). Thus, a cardiac myxoma originating in the mitral valve or LVOT should be removed immediately (13).
It is possible to obtain information regarding the attached portion and myocardial invasion of the myxoma through TEE, which can help to determine the surgical approach. In the case of general cardiac myxoma, the surgeon can remove the myxoma via an atrial approach, whereas a transaortic or transmitral approach is used for LV myxomas. In the case of LVOT myxoma in particular, a transaortic approach is appropriate to avoid the risk of intraoperative embolization (14).
Recently, noninvasive tests, such as an echocardiography, have been useful for evaluation and diagnosis of a myxoma and other cardiac masses (15). Although preoperative TTE is useful for diagnosing and evaluating an LV mass, TEE is more sensitive than TTE for obtaining more detailed information, including the morphology and attachment site of the mass. Preoperative TEE is advisable when TTE provides limited information and other tests, such as cardiac magnetic resonance imaging, are not performed because the surgical and anesthetic plan may differ among differential diagnoses.
In conclusion, although it could be particularly difficult to distinguish the LV mass which invades the mitral valve, intraoperative TEE is a useful tool for distinguishing a myxoma from other intracardiac masses, such as vegetation, cardiac thrombus or an accessory mitral valve tissue, in addition to clinical symptoms and signs.
Acknowledgements
This work was supported by clinical research grant in 2016 from Pusan National University Hospital.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- van der Heusen FJ, Stratmann G, Russell IA. Right ventricular myxoma with partial right ventricular outflow tract obstruction. Anesth Analg 2006;103:305-6. [Crossref] [PubMed]
- Burke A, Virmani R. Tumors of the Heart and Great Vessels (Atlas of Tumor Pathology. 3rd Series) (Vol. 16). Washington, DC: Armed Forces Institute of Pathology, 1996.
- Baek SH, Kim HY, Kim HJ, et al. Preoperative apical 5-chamber view transthoracic echocardiogram—the mass (white arrow) is protruding into the left ventricular outflow tract. Asvide 2017;4:118. Available online: http://www.asvide.com/articles/1428
- Baek SH, Kim HY, Kim HJ, et al. Preoperative apical 5-chamber view transthoracic echocardiogram—the protruding mass is causing left ventricular outflow tract flow acceleration. Asvide 2017;4:119. Available online: http://www.asvide.com/articles/1429
- Baek SH, Kim HY, Kim HJ, et al. Preoperative parasternal long axis view transthoracic echocardiogram—the mass (red arrow) looks like to be attached to papillary muscle (white arrow). Asvide 2017;4:120. Available online: http://www.asvide.com/articles/1430
- Baek SH, Kim HY, Kim HJ, et al. Preoperative parasternal short axis view transthoracic echocardiogram—the mass (in the circle) looks like to be attached to anterior mitral valve leaflet. Asvide 2017;4:121. Available online: http://www.asvide.com/articles/1431
- Baek SH, Kim HY, Kim HJ, et al. Intraoperative midesophageal left ventricular long-axis view transesophageal echocardiogram, showing the mass (black arrow) protruding into the left ventricular outflow tract during systole and occupying the entire lumen, resulting in left ventricular outflow tract obstruction. Asvide 2017;4:122. Available online: http://www.asvide.com/articles/1432
- Yoon JH, Kim JH, Sung YJ, et al. Cardiac myxoma originating from the anterior mitral valve leaflet. J Cardiovasc Ultrasound 2011;19:228-31. [Crossref] [PubMed]
- Burke A, Jeudy J Jr, Virmani R. Cardiac tumours: an update: Cardiac tumours. Heart 2008;94:117-23. [Crossref] [PubMed]
- Hesse B, Murphy RT, Myles J, et al. Images in cardiovascular medicine. A left atrial appendage thrombus mimicking atrial myxoma. Circulation 2006;113:e456-7. [Crossref] [PubMed]
- Restrepo CS, Largoza A, Lemos DF, et al. CT and MR imaging findings of benign cardiac tumors. Curr Probl Diagn Radiol 2005;34:12-21. [Crossref] [PubMed]
- Mahmoud HM, Moursi I. A rare case of a big left ventricular myxoma presenting with a cerebrovascular stroke. Egypt Heart J 2014;66:375-7. [Crossref]
- Samdarshi TE, Mahan EF 3rd, Nanda NC, et al. Transesophageal echocardiographic diagnosis of multicentric left ventricular myxomas mimicking a left atrial tumor. J Thorac Cardiovasc Surg 1992;103:471-4. [PubMed]
- Kawano H, Tayama K, Akasu K, et al. Left ventricular myxoma: report of a case. Surg Today 2000;30:1112-4. [Crossref] [PubMed]
- Alizade E, Karabay CY, Kilicgedik A, et al. A giant right atrial myxoma demonstrated by RT-3D transesophageal echocardiography and magnetic resonance imaging. Cardiol J 2011;18:320-1. [PubMed]


