Electromagnetic navigational bronchoscopy with dye marking for identification of small peripheral lung nodules during minimally invasive surgical resection
Introduction
Lung cancer continues to be the second most common cancer diagnosed among men and women in the United States and accounts for more deaths than any other cancer, with an estimate of 224,390 new cases and over 158,000 deaths for 2016 (1). At the time of diagnosis almost 70% of the patients have locally advanced or metastatic disease with an overall 5-year survival of approximately 15% (2). An early diagnosis and timely surgical resection is essential to improve patient survival and outcomes (3).
New screening programs for lung cancer using low-dose CT scan have shown an increase in early detection and diagnosis with the subsequent relative risk reduction in mortality (4). However, this has led to an increase in the detection of smaller lung lesions previously not detected with other imaging techniques. This can represent a challenge for thoracic surgeons who may need to localize these small, sometimes not palpable sub-centimeter lesions, in order to achieve a complete and successful surgical resection.
Current methods for localizing lung nodules include percutaneous coil or hook-wire placement and transthoracic CT-guided marking with methylene blue or radionuclide injection (5-9). Although, these techniques had shown good rates of success for the localization of lung nodules, the rate of complications, the time spent between the marking procedure and the surgical resection, and the exposure to ionizing radiation are greater when compared to ENB-guided marking.
Novel techniques have emerged to aid in the diagnosis and localization of these small peripheral lung lesions, reducing invasiveness and the risk of complications. Electromagnetic navigational bronchoscopy (ENB) is a novel technique that has proven itself useful for the diagnosis and localization of small peripheral lesions that may be difficult to palpate and locate during minimally invasive surgery (10). This technique offers a less invasive procedure combining conventional and virtual bronchoscopy with fewer rates of complications than with other more invasive techniques (11). ENB allows the guidance of diagnostic instruments to lung areas beyond the reach of conventional bronchoscopy and consists of four components; computer software that creates a three-dimensional virtual bronchoscopy reconstruction from CT scan images, an electromagnetic location board, a locatable sensor probe (the locatable guide) with an eight way steering mechanism which navigates the bronchial tree, and an extended working channel that can carry either the probe and bronchoscopic tools for biopsy and/or dye marking (12,13).
Previous studies have shown that the use of ENB with dye marking for the localization of small, non-palpable pulmonary nodules or ground-glass opacities (GGO) is a safe and feasible procedure (10,14-16). We believe that ENB-guided dye marking will aid in the localization of lung lesions that might be difficult to identify intra-operatively, due to factors such as small size, ground-glass rather than solid appearance, location away from the pleural surface and prior thoracic procedures, radiation or infection increasing the possibility of adhesions. Our initial experience with ENB-guided dye marking is presented in this report.
Methods
Institutional review board approval was granted for this study (IRB ID number H-33667). A retrospective review of all patients undergoing ENB between September 2012 and September 2016 at our institution was conducted. Clinical variables including age, gender, prior history of cancer, smoking status, lesion size and location, CT-scan characteristics, distance from pleural surface, surgical procedure and pathologic diagnosis were collected for analysis.
Seventeen patients with 19 nodules underwent ENB-guided dye marking for the localization of lung nodules with subsequent VATS or RATS resection. Due to the small size or predominately ground glass component of the lesions it was decided to proceed with ENB-guided dye marking for localization of the lung nodules followed by minimally invasive resection during the same procedure. Furthermore, due to the history of previous thoracic procedures, chest wall radiation and chronic inflammatory processes in five patients, we thought that the localization of these nodules with the use of ENB-guided dye marking was worthwhile in order to facilitate lesion recognition in a previously manipulated surgical field where adhesiolysis was likely to be required.
ENB Localization, dye marking and surgical procedure
ENB-guided dye marking of the 19 nodules was performed by the surgeon in the OR, immediately prior to VATS or RATS resection. The super Dimension software (version 6.3.9) and computer (Covidien, Minneapolis, MN) were used as part of the planning phase before the procedure. General anesthesia was induced and a single-lumen endotracheal tube was placed in the OR. Conventional bronchoscopy to the level of the segmental bronchi was performed in all patients. ENB was then performed, and navigation to the previously identified lesions during the planning phase was achieved. Intraoperative fluoroscopy was used to confirm placement of the locatable guide into the area of interest. The locatable guide was then removed leaving the extended working channel. A standard flexible bronchoscopic needle was passed through the extended working channel to the target lesion. The needle is primed with methylene blue before placing this in the extended working channel. Methylene blue (0.5–1 mL) was then injected directly into the lesion for pleural-based nodules, or peripheral to the lesion, but adjacent to the pleura for lesions deep to the pleural surface. It should be noted that the dye-marking procedure is only helpful for lesions in the outer 3rd of the lung that would be amenable to wedge or segmental resection.
After successful localization and marking of the 19 nodules, the single-lumen endotracheal tube was changed to a double-lumen endotracheal tube. Minimally invasive surgical resection was performed in sixteen cases and in one case it was decided to convert to thoracotomy due to the evidence of poorly formed fissures and incomplete deflation of the lung, which interfered with the execution of the procedure. Dense adhesions were present in five patients and adhesiolysis was performed in order to facilitate visualization and surgical resection.
Technical success was defined as the identification of dye within the lesion or close to it during minimally invasive resection, with pathologic confirmation of the lesion contained in the surgically resected specimen.
Results
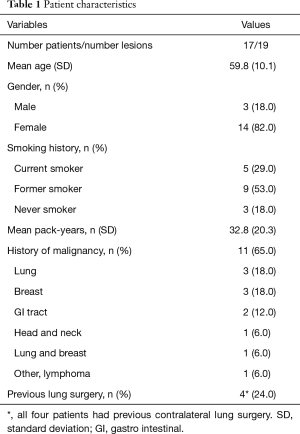
Seventeen patients with 19 nodules underwent ENB-guided dye marking to aid in lung nodule localization for subsequent VATS and RATS resection. Mean age was 59 years (SD ±10.1), and 82% (14/17) of the patients were female. Fourteen of 17 patients were current or former smokers (82%). Sixty-five percent of the patients had history of malignancy, and 25% had previous contralateral lung surgery (Table 1).
Full table
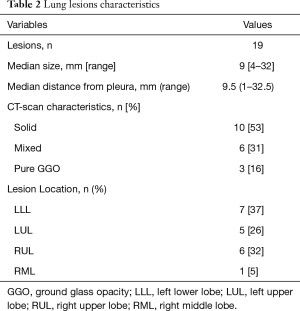
Fifteen patients had one lesion and two patients had two lesions that were navigated to and localized. Overall success rate for the identification of lung nodules after ENB-guided dye marking was 79% (15/19). The median lesion size was 9 mm (range, 4–32 mm) and the median distance from the visceral pleural surface to the center of the lesions was 9.5 mm (range, 1–40 mm). In all cases where adhesion takedown was required, the dye was clearly visualized within the nodules. Fifty-three percent (10/19) of the lesions were described as solid, and 44% (9/19) were mixed (6/9) and pure ground glass opacities (3/9). Sixty-three percent of the lesions (12/19) were located within 20 mm from the pleural surface, with a mean distance from the pleural surface to distal border of the lesions of 11 mm (SD 4.5 mm). Of these, 58% (7/12) were abutting the pleura. Anatomic locations of the lesions are detailed in Table 2.
Full table
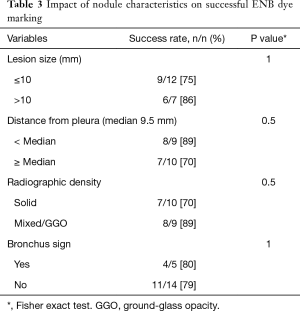
The success rate for the localization of lesions >10 mm was 86% (6/7), compared with a success rate of 75% (9/12) for lesions that were ≤10 mm (P=1.0). Eighty-nine percent of the lesions that were closer to the pleural surface (distance from pleural surface lower than median; 9.5 mm) were clearly identified after dye marking, compared to 70% success rate when the lesions were deeper in the lung parenchyma (distance from plural surface higher or equal than median; P=0.5). There was no significant difference in the success rate for the identification of solid compared to ground-glass/mixed lesions (Table 3).
Full table
Localization of lung nodules during minimally invasive resection
VATS or RATS resection was immediately performed after dye marking. In fifteen lesions the dye was clearly visualized and the lesion removed with a minimally invasive resection (technical success rate 79%). In two lesions the dye was not visualized, and in the remaining two nodules the dye extravasated into the pleural space with diffuse staining of the lung parenchyma interfering with nodule identification. Surgical resection and pathologic confirmation of the lung nodule within the surgical specimen was achieved in all cases. However one patient where the dye was not visualized, conversion to thoracotomy was performed due to the presence of poorly formed fissures and lack of atelectasis during VATS resection
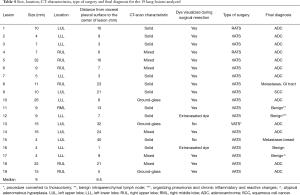
Fifteen patients underwent VATS resection and two patients had RATS. Malignancy was diagnosed in fifteen nodules. Of these, 87% (13/15) were diagnosed as primary lung cancers (12 adenocarcinomas and 1 squamous cell carcinoma), and two cases were metastatic lesions from gastrointestinal tract and breast primaries. In the remaining four nodules, a benign diagnosis was made and follow-up imaging confirmed resolution and no progression of findings (Table 4). There were no significant perioperative adverse events (in particular pneumothorax) after ENB-guided dye marking or surgical resection.
Full table
Discussion
The number of patients referred to thoracic surgeons for the evaluation of small lung nodules identified as part of new screening programs has increased over time. The correct approach for these small, usually sub-centimeter lesions, can be challenging since localization and palpation during minimally invasive resection is limited.
Our experience with the use of ENB and dye marking for the localization of small, peripheral lesions with subsequent VATS or RATS resection showed that this approach may be of great utility for thoracic surgeons increasing workflow and avoiding additional procedures and inconveniences for patients.
Our high success rate for the identification of lung nodules with the use of ENB-guided dye marking demonstrates that this procedure should be considered a good option for approaching sub-centimeter or larger ground-glass lung lesions for minimally invasive resection.
ENB-guided dye marking is safe and feasible for the localization of small peripheral lung nodules for subsequent minimally invasive surgical resection (10,14,15). Recent studies have shown high success rates for the localization of sub-centimeter lesions with low rate of complications (15,16). Krimsky and colleagues (14) reported a success rate of 81% for the localization of 21 nodules (mean size 13.4 mm; range 7–30 mm) during VATS and RATS resection after the injection of indigo carmine and methylene blue using ENB. Similarly, Bolton et al. (10) reported a high success rate for the localization and subsequent RATS resection in 16 patients without adverse events related to ENB dye injection. In more recent studies, Awais and colleagues (15) showed successful results in 29 patients that underwent ENB dye marking with subsequent VATS and RATS resection. In this study with 33 lesions (median size 10 mm; range, 4–27 mm), the pleural dye was visualized and a successful resection was achieved in all cases. Likewise, Marino et al. (16) reported a 97% success rate for the identification of lung nodules after ENB dye marking in 70 patients without conversion to thoracotomy or any adverse events related to ENB-guided marking.
Although, our success rate was not as high as previously reported, we included smaller lesions compared to previous studies, which were successfully identified during VATS or RATS resection. Moreover, five of our patients required adhesiolysis, and in all these cases dye marking was successful, allowing a minimally invasive resection.
Interestingly, the two lesions that failed to be identified after dye marking were the ones located more deeply into the lung parenchyma (Table 4). In one of these cases a conversion to thoracotomy was necessary due to the inability to visualize the dye and because the lung was not deflating well during VATS resection, which made the performance of the procedure more difficult. However, the presence of the dye was later confirmed near the nodule during frozen section analysis. The other lesion that was not identified during VATS resection was a 4 mm lesion located at 40 mm from the pleural surface. We believe that the small size and location of this lesion may explain the inability to visualize it during minimally invasive resection.
In two other lesions the dye extravasated into the pleural space interfering with their localization. One of these lesions was in contact with the pleural surface, which could explain why the dye was found in the pleural space. Care must be taken to avoid injecting into the pleural space. The other lesion was also close to the pleural surface (3 mm from proximal border) increasing the risk of dye extravasation. Notably, and despite the failure in localizing these four lesions after ENB-guided dye marking, a complete surgical resection and a pathologic diagnosis was achieved in all cases. In two cases where the dye was not visualized, nodularity was palpable by the surgeon during the procedure helping in the location and complete resection. In the remaining two cases it was decided to proceed with an extended segmentectomy and a wedge resection according to the segmental anatomy discerned by the preoperative CT scan.
We found that the use of ENB-guided dye marking for the localization of these peripheral lung nodules is useful in patients that require adhesion take down during VATS or RATS resection. Dense adhesions within the pleural space were found in five of our patients, requiring extensive adhesion take down before surgical resection. We believe that ENB-guided dye marking was of great utility in these cases were the localization of the nodules was obscured by the changes found in a previously manipulated and/or irradiated surgical field.
We conclude that the use of ENB-guided dye marking for the localization of small peripheral lung nodules followed by minimally invasive resection is a safe and feasible procedure with a high rate of success. Additional studies are required in order to establish well-defined selection criteria for patients who may benefit from this less invasive procedure. An advantage compared to other localization techniques, is that that thoracic surgeon can perform this, immediately prior to resection in the same setting. This improves workflow and avoids the need for additional procedures and inconvenience to the patient.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by Institutional review board (IRB ID number H-33667).
References
- Cancer Facts & Figures 2016. Atlanta: American Cancer Society; 2016. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2016.html
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- International Early Lung Cancer Action Program Investigators., Henschke CI, Yankelevitz DF, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763-71. [Crossref] [PubMed]
- National Lung Screening Trial Research Team., Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. [Crossref] [PubMed]
- Lizza N, Eucher P, Haxhe JP, et al. Thoracoscopic resection of pulmonary nodules after computed tomographic-guided coil labeling. Ann Thorac Surg 2001;71:986-8. [Crossref] [PubMed]
- Chen S, Zhou J, Zhang J, et al. Video-assisted thoracoscopic solitary pulmonary nodule resection after CT-guided hookwire localization: 43 cases report and literature review. Surg Endosc 2011;25:1723-9. [Crossref] [PubMed]
- Bertolaccini L, Terzi A, Spada E, et al. Not palpable? Role of radio-guided video-assisted thoracic surgery for nonpalpable solitary pulmonary nodules. Gen Thorac Cardiovasc Surg 2012;60:280-4. [Crossref] [PubMed]
- Doo KW, Yong HS, Kim HK, et al. Needlescopic resection of small and superficial pulmonary nodule after computed tomographic fluoroscopy-guided dual localization with radiotracer and hookwire. Ann Surg Oncol 2015;22:331-7. [Crossref] [PubMed]
- Lin MW, Tseng YH, Lee YF, et al. Computed tomography-guided patent blue vital dye localization of pulmonary nodules in uniportal thoracoscopy. J Thorac Cardiovasc Surg 2016;152:535-44.e2. [Crossref] [PubMed]
- Bolton WD, Howe H 3rd, Stephenson JE. The utility of electromagnetic navigational bronchoscopy as a localization tool for robotic resection of small pulmonary nodules. Ann Thorac Surg 2014;98:471-5; discussion 475-6. [Crossref] [PubMed]
- Eberhardt R, Anantham D, Ernst A, et al. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med 2007;176:36-41. [Crossref] [PubMed]
- Weiser TS, Hyman K, Yun J, et al. Electromagnetic navigational bronchoscopy: a surgeon's perspective. Ann Thorac Surg 2008;85:S797-801. [Crossref] [PubMed]
- Port J, Harrison S. Electromagnetic navigational bronchoscopy. Semin Intervent Radiol 2013;30:128-32. [Crossref] [PubMed]
- Krimsky WS, Minnich DJ, Cattaneo SM, et al. Thoracoscopic detection of occult indeterminate pulmonary nodules using bronchoscopic pleural dye marking. J Community Hosp Intern Med Perspect 2014.4. [PubMed]
- Awais O, Reidy MR, Mehta K, et al. Electromagnetic Navigation Bronchoscopy-Guided Dye Marking for Thoracoscopic Resection of Pulmonary Nodules. Ann Thorac Surg 2016;102:223-9. [Crossref] [PubMed]
- Marino KA, Sullivan JL, Weksler B. Electromagnetic Navigation Bronchoscopy for Identifying Lung Nodules for Thoracoscopic Resection. Ann Thorac Surg 2016;102:454-7. [Crossref] [PubMed]


