Color-Doppler sonography patterns in endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal lymph-nodes
Introduction
Endobronchial ultrasound guided transbronchial needle aspiration (EBUS-TBNA) is commonly used for the pathological diagnosis in patients with mediastinal lesion; although this procedure has been used for mediastinal masses and lymph node metastases from extra-thoracic tumors, the lung cancer staging covers the main indication (1-3). The recent revised European Society of Thoracic Surgeons guidelines for preoperative mediastinal lymph-node staging for non-small-cell lung cancer (NSCLC) recommend EBUS-TBNA as a reliable first-choice, limiting more invasive surgical staging after inconsistent cytological results from EBUS-TBNA (4). The accuracy of EBUS-TBNA is considered high: a meta-analysis published in 2015 established the pooled sensitivity at 0.83 (95% CI: 0.79–0.87) and the pooled specificity at 100% (5). Several authors have tried to outline characteristic ultrasound criteria for malignant mediastinal lymph-node identification to maximize the TBNA accuracy; such criteria included the nodal appearance, vascularization and elasticity (6-8). The aim of the present study was to assess the vascular pattern classification of mediastinal lymph-nodes during EBUS-TBNA proposed by Takahiro Nakajima and colleagues in 2012 (6).
Methods
The current single-center prospective cohort trial was conducted between June 2015 and September 2016 in a university hospital setting after local ethical committee approval. The main end-point of the study was the predictive value of color-Doppler pattern for EBUS-TBNA pathological diagnosis. All patients referred to our institution for suspected non-lymphomatous malignancy in thoracic lymph-nodes underwent a conventional diagnostic work-up including contrast-enhanced chest computer tomography (CT) and positron emission tomography with integrated CT (PET-CT). Lymph-nodes with a short axis diameter of more than 10 mm on chest CT were considered suspected for malignancy; maximum standardized uptake value greater than 2.5 defined suspected lymph-nodes on PET-CT scan. The trial inclusion criteria were: adulthood, positive mediastinal lymph-nodes or positive hilar lymph-nodes with negative mediastinal nodes; exclusion criteria were severe co-morbidity. Written informed consent was obtained from each patient at hospital admission. The enrollment stopped on March 2016 to achieve at list six months follow-up for each patient.
EBUS-TBNA was performed in a day-hospital setting; local anesthesia and conscious sedation were administered following British Thoracic Society guidelines. A flexible bronchoscope (BF-UC180F, Olympus Optical Co Ltd., Tokyo, Japan) was used to guide a dedicated 22 G needle. Three aspirations were done at each node; if more than one node was detected, the aspiration was applied at all suspected nodes. Rapid on-site evaluation was not used. Samples were fixed with formalin and sent for pathological and molecular examinations. EBUS-TBNA cytological results were classified inadequate in presence of an excess of red blood cells, or if only bronchial cells were identified, or if the concentration of lymphocytes was not representative of lymph node tissue.
Lymph-node color-Doppler patterns were categorized by two operators on EBUS findings following the classification proposed by Nakajima et al.: grade 0, almost no blood flow; grade I, few vessels; grade II, few punctiform flow signals and/or few small vessels; grade III, rich flow. The blood flow from the bronchial arteries (BA inflow sign) is defined positive if a blue signal toward a lymph-node was identified (6). Lymph-nodes with grade 0 or I were considered benign; conversely, lymph-nodes with grade II or III were considered malignant. Positive BA inflow sign was considered as an indication of malignancy regardless of the degree described above. Inadequate pathological results were excluded from the analysis, malignant and benign pathological results were confirmed by surgery or clinical follow-up.
Data are presented with numbers (percentages) for categorical variables and median (95% confidence interval) for continuous variables. The diagnostic sensitivity, specificity, positive predictive value (PPV), negative predicted value (NPV) and accuracy were calculated using standard definitions. A P value <0.05 was considered significant. Statistical analyses were undertaken using MedCalc Software version 14.8.
Results
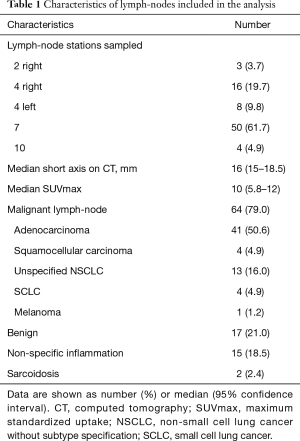
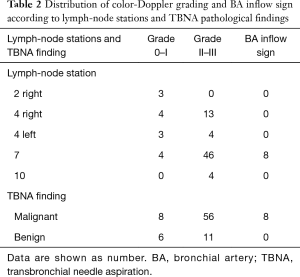
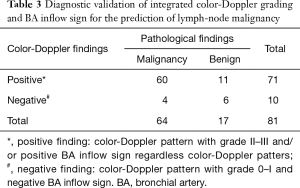
Seventy-five patients were enrolled in the study, the rate of male gender was 64%, the median age was 66 years (64–69 years); 85% of patients had primary lung cancer and 15% had an extra-thoracic malignancy. EBUS-TBNA was performed in 87 lymph-nodes (rate 1.16 per patient); 6 lymph-nodes had inadequate samples and were excluded from the analysis; 64 nodes were confirmed as malignant and 17 nodes had benign diagnoses. The median duration of the procedure was 30 minutes (95% CI: 24.6–35). No severe complications occurred. Characteristics of lymph-nodes included in the analysis are listed in the Table 1. Distribution of color-Doppler grading and BA inflow sign according to lymph-node stations and TBNA pathological findings are shown in Table 2. All EBUS-TBNA pathological findings were confirmed by follow-up or surgery. The sensitivity, specificity, PPV, NPV, and diagnostic accuracy of integrated color-Doppler grading and BA inflow sign for the prediction of lymph-node malignancy were 0.93, 0.64, 0.84, 0.6 and 0.81 respectively (Table 3).
Full table
Full table
Full table
Discussion
EBUS-TBNA is a commonly used endoscopic procedure that have a high accuracy for detecting metastasis to hilar and mediastinal lymph nodes. The discrimination between malignant and benign lymph-nodes by EBUS essentially follows the dimensional criterion generally used for computed tomography. Once a suspected lymph-node is identified, the comparison between the short-axis measurement with EBUS and CT enhance the precision of sampling; in addition, shape and echogenicity could help the targeting (9). Notwithstanding, a more specific prediction pattern of the malignant involvement could reduce unnecessary lymph-node sampling during EBUS-TBNA.
Considering that sonographic features have been shown to be useful for the prediction of malignant lymph-nodes during endoscopic ultrasound (EUS), Fujiwara and colleagues proposed a classification of lymph-node layout during EBUS in 2010 (6). Such morphological classification took into account several sonographic features: size, less or more than 10 mm; shape, oval or round; margin, distinct or indistinct; echogenicity, homogeneous or heterogeneous; central hilar structure, present or absent; coagulation necrosis sign, present or absent. The following patterns were considered suggestive for malignancy: short axis of more than 10 mm, round profile, distinct margin, heterogeneous echogenicity, absence of central hilar structure, or presence of coagulation necrosis sign. Statistical analysis defined the lymph-node shape, margin, echogenicity, and coagulation necrosis sign as independent predictive factors for malignancy. This detailed morphological classification is certainly useful in clinical practice but suffers severely from subjective interpretation.
Trosini-Désert and colleagues published the use of elastography during EBUS in 2013 (10). Ultrasound elastography has formerly been applied as an external procedure for breast and other tumors since malignancy has lower elasticity than healthy tissues. Dedicated EBUS software provides the evaluation of elastic properties of mediastinal elements by color-coding patterns: color blue indicates hard tissues, generally malignant; color green is typically referred to tissues with intermediate elasticity as fat-fibrous tissue; color red designates soft tissue, generally vascular structures. Izumo and colleagues identified three lymph-node color patterns which are correlated with a progressive increasing probability to obtain a malignant result from TBNA: type 1, predominantly non-blue, type 2, partially blue; type 3, predominantly blue (11). The EBUS software makes also possible the elasticity quantification and the calculation of quotient between two different areas selected by the operator; such numeric parameter is commonly known as stain ratio. The best strain ratio cut-off point has been calculated by Rozman and colleagues: strain ratio values ≥8 identifies lymph-node malignancy with an accuracy of 86.25% (12). Our personal experience is that obtaining a good and consistent elastographic picture could be sometime quite demanding and time consuming, especially if the strain ratio was required.
It is well known that color-Doppler sonography is useful in the differentiation of benign from malignant cervical lymphadenopathy (13). A significant positive correlation was also observed between color-Doppler patter and level of vascular endothelial growth factor in breast cancer, suggesting a possible relationship among Doppler signal, angiogenesis and tumors aggressiveness (14). Considering the distortion of the vascular pattern caused by neoplastic infiltration of a lymph-node, the identification of mediastinal metastatic nodes with EBUS is possible. Nakajima and colleagues worked on this issue and proposed a classification articulated in four grades plus a specific sign (6). We assessed such classification in our clinical practice with the current prospective trial. The study cohort included patients with mediastinal or hilar lymphadenopathy highly suspected for malignancy; finally, cancer prevalence in the target area was fairly high (79%). The TBNA adequacy rate was sufficient (93%) while the cytological diagnoses from adequate samples were confirmed with surgery and/or follow-up in all cases. Our results confirmed the usefulness of color-Doppler patterns in the identification of mediastinal lymph-node suspected for malignancy: color-Doppler patters with grade 2/3 or positive BA inflow sign determined a PPV greater than 80%. This positive result does not mean that the color-Doppler imaging can replace the TBNA, but simply it can guide the operator in choosing the node to be sampled with the best chance for significant findings. We found the execution of the color-Doppler scan extremely fast and easy to be interpreted; this could depend on fact that operators normally use the Doppler sonography for the secure identification of vascular structures.
The current study has some limitations: firstly, this trial did not have a control arm; large randomized studies should be necessary to definitively validate the Nakajima vascular pattern classification. The second limitation was related to the possible subjective interpretation of the color-Doppler finding; to decrease personal rendering each test was ran by two expert operators. Points of strength are that this study was prospective, all EBUS-TBNA procedures were performed by the same team of two expert bronchoscopists, and the cytological analyses were conducted by pathologists dedicated to thoracic diseases, reducing in this way the learning curve bias. Moreover, the study has no missing data and a uniform mode of management.
Conclusions
EBUS-TBNA is an accurate and minimally invasive tool used for mediastinal lymph-node diagnosis. To enhance its accuracy a number of sonographic ancillary techniques have been proposed; among those methodologies, the color-Doppler sonography seems to be the fastest, most reproducible and effective one. The present prospective study validates the color-Doppler vascular pattern classification proposed by Nakajima and colleagues. In our setting this classification was really helpful for the identification of suspected mediastinal lymph-node reaching an accuracy of 81%.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The current single-center prospective cohort trial was conducted between June 2015 and September 2016 in a university hospital setting after local ethical committee approval. Written informed consent was obtained from each patient at hospital admission.
References
- Herth FJ, Eberhardt R, Vilmann P, et al. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax 2006;61:795-8. [Crossref] [PubMed]
- Koh MS, Ong TH, Phua GC, et al. Feasibility of endobronchial ultrasound in mechanically ventilated patients. Ann Acad Med Singapore 2014;43:238-40. [PubMed]
- Nosotti M, Tosi D, Palleschi A, et al. Transbronchial needle aspiration under direct endobronchial ultrasound guidance of PET-positive isolated mediastinal adenopathy in patients with previous malignancy. Surg Endosc 2009;23:1356-9. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Ge X, Guan W, Han F, et al. Comparison of Endobronchial Ultrasound-Guided Fine Needle Aspiration and Video-Assisted Mediastinoscopy for Mediastinal Staging of Lung Cancer. Lung 2015;193:757-66. [Crossref] [PubMed]
- Fujiwara T, Yasufuku K, Nakajima T, et al. The utility of sonographic features during endobronchial ultrasound-guided transbronchial needle aspiration for lymph node staging in patients with lung cancer: a standard endobronchial ultrasound image classification system. Chest 2010;138:641-7. [Crossref] [PubMed]
- Nakajima T, Anayama T, Shingyoji M, et al. Vascular image patterns of lymph nodes for the prediction of metastatic disease during EBUS-TBNA for mediastinal staging of lung cancer. J Thorac Oncol 2012;7:1009-14. [Crossref] [PubMed]
- Janssen J, Dietrich CF, Will U, et al. Endosonographic elastography in the diagnosis of mediastinal lymph nodes. Endoscopy 2007;39:952-7. [Crossref] [PubMed]
- Groth SS, Andrade RS. Endobronchial ultrasound-guided transbronchial needle aspiration for mediastinal lymph node staging in non-small cell lung cancer. Semin Thorac Cardiovasc Surg 2008;20:274-8. [Crossref] [PubMed]
- Trosini-Désert V, Jeny F, Taillade L, et al. Bronchial endoscopic ultrasound elastography: preliminary feasibility data. Eur Respir J 2013;41:477-9. [Crossref] [PubMed]
- Izumo T, Sasada S, Chavez C, et al. Endobronchial ultrasound elastography in the diagnosis of mediastinal and hilar lymph nodes. Jpn J Clin Oncol 2014;44:956-62. [Crossref] [PubMed]
- Rozman A, Malovrh MM, Adamic K, et al. Endobronchial ultrasound elastography strain ratio for mediastinal lymph node diagnosis. Radiol Oncol 2015;49:334-40. [Crossref] [PubMed]
- Ahuja AT, Ying M, Ho SS, et al. Distribution of intranodal vessels in differentiating benign from metastatic neck nodes. Clin Radiol 2001;56:197-201. [Crossref] [PubMed]
- Wang Y, Dan HJ, Fan JH, et al. Evaluation of the correlation between colour power Doppler flow imaging and vascular endothelial growth factor in breast cancer. J Int Med Res 2010;38:1077-83. [Crossref] [PubMed]


