Zenker’s diverticulum: flexible versus rigid repair
Introduction
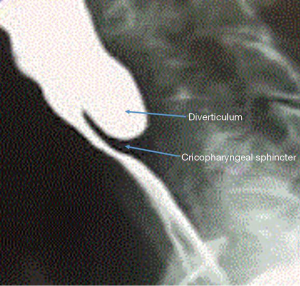
Zenker’s diverticulum (ZD) or pharyngeal pouch is a rare esophageal disorder with an annual incidence of just 2 per 100,000. It is a pulsion diverticulum that is likely the result of poor coordination between the pharyngeal and upper esophageal sphincter contractions at an area of anatomical weakness. This area known as the Killian triangle, occurs at the origin of the inferior pharyngeal constrictor and cricopharyngeus muscles. Pressure increases and inadequate opening of the upper esophageal sphincter create a hernia of mucosa. Alterations in tone and motility of the upper esophageal sphincter may be the result of GERD (1). The pharyngeal pouch is a false diverticulum of mucosa, but the common wall or septum between the diverticular lumen and the esophageal lumen is full thickness, including the cricopharyngeus muscle (Figure 1). Multiple surgical and endoscopic treatments are acceptable for ZD. No randomized trials exist by which to establish superiority of one approach.
Indications for repair
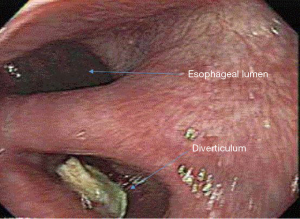
ZD is usually diagnosed by either contrast esophagram or upper endoscopy to evaluate esophageal symptoms (Figure 2). The classic presentation is that of an elderly patient with dysphagia, halitosis, regurgitation, or aspiration. However some may be identified incidentally in patients experiencing GERD or laryngopharyngeal reflux symptoms. Unfortunately, many patients with ZD are not identified or not offered surgical therapy until the disease is advanced, sometimes to the point of nutritional compromise. ZD tend to manifest in elderly, debilitated patients, leading to higher risk and worse surgical morbidity and mortality. Malnutrition should be quantified and treated before and after surgery for ZD to prevent complications and decrease mortality (2). Despite this, surgical treatment is successful in relieving symptoms in 80% to 100% of patients (3).
History
Open transcervical diverticulectomy was first performed in 1885, and is the “gold standard” for repair of ZD. The surgical techniques and strategies have changed, some falling in and out of favor over time. Diverticulectomy was later modified to a two stage procedure, with the first stage to elevate the diverticulum and the delayed stage for resection. This was found to reduce the associated mortality rate and was met with wide reception, eventually by 1950s single stage procedure was en vogue again due to difficulty employing the two stage approach in elderly debilitated patients. With routine use of antibiotics and improved postoperative care, and the mortality rate fell to reported 1.2% (1,4). Endoscopic techniques which were first attempted in the early 20th century were initially unpopular due to high risk of mortality and morbidity. In the 1960s a modification of the rigid esophagoscope allowed for improved technique and outcomes, then the introduction of staplers in 1990s led to increased adoption of endoscopic techniques. Flexible endoscopists took their turn as well in the 1990s, adding to the pool of viable minimally invasive strategies treatment options.
Evolution of technique
Surgical options include a variety of tools and techniques and to accomplish some combination of diverticulectomy, diverticulopexy, and cricopharyngeal myotomy via a left neck incision. Historically, there was some controversy over the necessity of myotomy as diverticulectomy or diverticulopexy alone in open surgery has been shown to have good results. However, the addition of myotomy was shown to improve results and minimize resection leaks, and is now considered optimal for any pharyngeal pouch procedure (5). Over the years of open surgery for ZD, it became generally recommended that small pouches 1 cm could be treated with myotomy alone, 1–4 cm pouches require myotomy and diverticulum suspension, and larger warranted diverticulectomy and myotomy though variation in surgical strategy is common (6).
Open surgery
In the modern era (last 10 years), open transcervical surgical treatment of ZD has an overall morbidity of 10.5%, mortality of 0.6% as tabulated in a review of 2,826 patients from 41 studies. The open approach varied in these studies, with no less than seven variations of surgical technique. Overall, the risk of serious complications was low, with 0.2% mediastinitis. 3.3% leak or perforation, 3.3% recurrent laryngeal nerve injury, and 1.8% cervical infection (7).
Some specialists consider the open transcervical approach the only option for small pouches 1 cm or smaller, or for very large diverticula (8). Open operations are considered by some to be the treatment of choice for young healthy patients because of more durable long-term results. Excision of the pouch sac has also been sometimes recommended in younger patients, patients less than 65 years, and for size considerations, to mitigate the risk of both recurrent symptoms and development of carcinoma. If endoscopic diverticulotomy is performed, then long-term patient symptom follow-up is to be advocated for carcinoma surveillance, though the incidence of cancer in the un-resected ZD pouch is very low (2,9).
A range of open and endoscopic approaches are generally accepted to be effective for relief of symptoms related to ZD. The benefits of less invasive endoscopic approaches are desirable and they have become more prevalent with time. Mortality rates are recorded as 0.3% for stapled and 0.2% for non-stapled rigid endoscopic procedures. No deaths have been reported for flexible endoscopic treatment of ZD to date (7).
For all types of endoscopic surgery for ZD, the main principle is division of the diverticular septum common wall, including complete myotomy of the cricopharyngeal muscle bar. This restores the diverticular pouch continuity with the esophageal lumen. While cricopharyngeal myotomy alone does not truly restore normal anatomy, it usually achieves symptomatic relief and emptying of the pharyngeal pouch.
Rigid endoscopic surgery
ENT specialists were first to develop transoral treatments for ZD. Mosher in 1917 first described transoral treatment of the ZD, dividing the common diverticular wall utilizing an endoscope. The unfortunate complication was mediastinal sepsis and the technique fell out of favor due to high morbidity and mortality. Dohlman in 1960 reported 30-year experience with endoscopic diverticulotomy using electrocautery to divide the common wall utilizing a rigid scope (10). Van Overbeek modified this technique utilizing the CO2 laser which improved visualization and allowed precision and control (11). Collard was first to utilize an endoscopic stapler to divide the common wall and described this technique in 1993 (12). As a whole, transoral techniques have become accepted as safer, faster, less invasive, and less costly compared to open. The rigid transoral stapling technique in particular has had favorable results and has become accepted as the minimally invasive standard of care and alternative to open transcervical surgery. In the US, rigid transoral techniques, with or without endoscopic visualization, are performed mostly by ENT surgeons, using stapling devices, CO2 laser, or other dissection techniques (13).
Surgical technique. Rigid endoscopic procedures require the use of a diverticuloscope, either Dohlman or Weerda types are utilized (Figure 3). The placement is similar to that of a rigid esophagoscope, but these have an apparatus that allows the scope to be fixed to the chest once the desired anatomical view is obtained. The procedure requires general anesthesia, as neck hyperextension is mandatory. Once the optimal view is obtained and the scope secured in position, the esophagus is cleared of food and debris. The diverticulum is identified and an endo GIA stapler of appropriate length is selected. As current staplers don’t staple and cut all the way to the tip, these are sometimes modified by the surgeon by cutting down the blade inserted in the diverticulum for a more complete division (Figure 4). The anvil limb is placed into the diverticular pouch and the cartridge is placed into the esophageal lumen. The stapler is fired, dividing the septum and sealing the edges to create a “delta” shaped anastomosis.

Non-stapled devices utilized for rigid endoscopic treatment of ZD include electrocautery, CO2 laser, and more recently Harmonic or LigaSure dissectors (14,15). Using similar diverticuloscope setup, these tools are used to divide the common wall septum of the diverticulum. With these techniques, the feared complication is unsealed edges, leading to leak and mediastinal sepsis, as well as the potential for bleeding. The improvement of clips and biologic glues for sealing the edges has renewed interest in these techniques.
Rigid endoscopic treatment for ZD requires careful consideration of the individual’s anatomy. Anatomical variations of the head and neck such as malocclusion, cervical kyphosis or fixation, retrognathia, large tongue, or dental abnormalities can increase the likelihood of failure of this technique. Patients should be carefully screened for these prior to attempting the procedure and for tolerance of neck hyperextension. Even with pre-procedure screening, 5.6% of cases fail rigid endoscopic treatment attempts due to anatomic difficulty, and traditionally are converted to open surgery, though flexible endoscopic treatment has potentially become an alternative if the surgeon is able to perform it.
Size of the diverticulum comes into play when deciding on rigid endoscopic treatment technique. For the stapled technique, it is optimal for treatment of ZD of least 2 cm in order for the stapler to properly engage and divide the common wall adequately. Large diverticula may pose an equally difficult challenge. Just identifying and isolating a diverticulum less than 2 cm with the rigid scope can be challenging, and may require more complex maneuvers to complete the myotomy appropriately to avoid a persistent pouch and recurrent symptoms (8). Applying traction sutures to the septum to pull it deeper into the stapler is one strategy that has been used successfully. Sutures are placed via the endoscope with either a laparoscopic needle driver, or with the Endo Stitch endoscopic suturing device (Covidien, Ireland), either of which may be challenging maneuvers. The traction suture assisted stapled technique does raise concern for increased risk of perforation. For these reasons, some surgeons consider the small diverticulum an indication for open or flexible endoscopic repair to avoid these difficulties while achieving and adequate and complete myotomy. A residual bridge after stapling can be divided with laser or other precision dissecting device (Figure 5). For very large diverticula, multiple staple loads can be used to completely divide the full length of the septum.
Recurrence of symptoms after prior open or endoscopic intervention for ZD is not a contraindication to rigid endoscopic stapling or other endoscopic treatment modalities.
Yuan reviewed multiples studies of rigid endoscopic ZD treatments, and broke results down by device. The Dohlman procedure utilizing electrocautery via the rigid diverticuloscope is associated with a 0.2% mortality, and an overall complication rate of 7.8% on average of 485 patients in nine studies. However, the overall complication rate was as high as 18% in some studies. Overall electrocautery was associated with 2.9% subcutaneous emphysema, and about 2.1% developed mediastinitis. Symptomatic relief was successfully achieved in 90.6–92.5% without recurrence between 10 and 42 months of follow up in various studies (7).
Dohlman’s procedure with electrocautery was eventually largely replaced by CO2 laser and stapler techniques. Of 1,060 patients from 19 studies for CO2 laser the overall complication rate was 9.3%, with a mortality rate of 0.2%. Of these patients 3% developed subcutaneous emphysema, 1.3% resulted in mediastinitis, 1.1% developed salivary fistula, and bleeding was notable in 1%. In follow up, 90.6–93% of patients achieved satisfactory symptom relief, and 3.9% developed symptoms suspicious for recurrence. It was noted that CO2 yielded less pain and faster oral intake the other procedures (16).
Rigid endoscopic stapling was reviewed in 44 studies including 1,800 patients. Overall the rate of complications was 7.1%, and overall mortality was 0.3%. Specific complications include 2% dental injury, 1.6% mucosal injury, 1.6% perforation. Conversion to open (failure of the rigid endoscopic approach) was required in 5.6%. In follow up, 92% of patients achieved symptomatic recurrence in 27 months, about 10% were found to have recurrence of symptoms in 32 months (7). Higher leak rate was associated with multiple staple fires for larger diverticula (17).
Bonavina and colleagues reported their experience with transoral stapling for ZD in 100 patients. Complications occurred in 4%, with no mortality. Long term success for median 63 months was achieved in 76% of patients. Patients of more advanced age had greater success rate. Use of traction sutures also improved the success rate. Twenty-four percent of patients had recurrent symptoms. These patients were more likely to have had a small <3 cm initial ZD, and were generally younger. Five went on to redo stapling and 3 had open surgery completed safely (18).
Combined procedures, using stapled diverticulotomy and CO2 laser dissection have also been proposed. This yielded 87% short term symptom improvement and 2.6% complication rate with reported improved hemostasis and lower leak rate.
Hondo and colleagues reported twenty cases of rigid endoscopy using the Ultrasonic coagulation scissors with equivalent symptom relief to the stapler assisted technique, with only one (5%) complication rate compared to 17.9% for the stapler group. The Harmonic was found to be useful for ZD 2cm or smaller, and the increased complication rate for the stapler group was attributed to larger average size of ZD. This device was faster and caused less tissue damage when compared to electrical current in an animal model (19).
Bipolar sealing devices have also been described in a handful of cases, with similar results (14,20).
Benefits of the rigid endoscopic surgical treatments of ZD are apparent. The procedure is suggested to be simple, though it is obvious that there is a learning curve associated with navigating the rigid diverticuloscope. The rigid endoscopic approach allows shorter operative time and faster recovery and return to normal diet compared to open procedures. There is decreased risk nerve injury and associated vocal cord paralysis. It is safe and easy in re-operative cases. About 90% of patients achieve symptomatic relief overall, usually with a single treatment.
Flexible endoscopic surgery
Flexible endoscopic surgery for ZD was first described in 1982 (21). This technique is more frequently utilized by gastroenterologists or surgical endoscopists. First case series for flexible endoscopy were presented in 1995 by Mulder (22,23). Historically, flexible endoscopic therapy for ZD was reserved for patients deemed to be poor surgical candidates. Reasons for this decision included comorbidities, intolerance of anesthesia, inability to accommodate rigid scope due to inability to hyperextend neck or other anatomical difficulties.
This technique may be performed utilizing various devices including Argon cautery, hook knife, needle knife, Triangle tip knife, bipolar or monopolar forceps (13,24-26) (Figure 6). This has become the surgical treatment of choice at our institution for ZD. Prophylactic antibiotics are given. General anesthesia is preferred for the ease of the surgeon, but the procedure can be safely performed with monitered sedation provided by an anesthesiologist. The surgeon uses a flexible, high definition gastroscope. Some may choose to use a soft diverticuloscope (modified overtube) according to preference but its use is not mandatory (in our practice the diverticuloscope is omitted). The tip of the gastroscope is fitted with a clear cap which aids in exposure and maintaining a clear lens (Figure 7). CO2 is used exclusively for insufflation in case of micro-perforation as it is absorbed by the soft tissues much more readily and causes less discomfort. The hook-knife cautery may be preferred, for the benefit of precision cutting and teasing out each muscle fiber. The common wall between the diverticulum is identified and the midline is marked by cautery burns down both the pouch and esophageal sides. The common wall is then divided including mucosa and muscle (cricopharyngeal sphincter) down to the tip of the diverticulum (Figure 8). Bleeding of the muscle is controlled with dilute epinephrine solution, or cautery (27). Closure of the edges of the resulting defect is usually recommended to minimize post procedure leaks and bleeding but is sometimes not done by some practitioners. Traditionally, endoscopists were conservative with the extent of the common wall division, for fear of freely perforating into the neck with subsequent risk of cervical infection or mediastinitis. This sometimes led to the same problem as seen with transoral stapling—residual pouch or incomplete myotomy—with the possibility of residual or recurrent symptoms. Based on our experience with NOTES and POEM, we have modified our procedures over the last 5 years to eliminate this problem. We now take the common wall division to the very tip of the diverticulum and then deliberately advance the endoscope between two layers of mucosa and into the neck. This allows us to extend the myotomy well onto the esophageal wall, usually for at least 5–10 mm beyond the diverticulum tip. This modification necessitates a secure closure of the mucosal defect. Mucosal closure can almost always be adequately performed with standard endoscopic clips. Closure is started at the apex (point most distal of the esophageal and diverticulum mucosal incision) and then continued up each side, approximating esophageal to diverticular mucosa (Figure 9). The endoscopic Overstitch suturing device (Apollo Endosurgery, Austin, TX) may be used for difficult closures. Postoperatively all patients get an esophagram to exclude leaks and then are started on a liquid/puree diet and discharged to home. A full liquid or pureed diet is recommended for 1 week to avoid clip dislodgement. Using this transesophageal extended myotomy technique, our dysphagia rate has decreased from 12% with standard technique, to less than 5% (28).
Flexible endoscopy cricopharyngeal myotomy for ZD is a useful alternative to rigid endostapling. Long-term data for this technique are sparse and studies are heterogeneous (28). A growing number of centers are building experience with this technique in the US, Asia, and Europe.
Yuan reviewed 472 patients in 12 studies of flexible endoscopic treatment of ZD. The overall complication rate was 15%, and mortality 0%. Specific complications cited include cervical emphysema 6%, perforation in 4%, notable bleeding in 3%. Reported rates of recurrence of symptoms were widely variable, from 0% in 20 months follow up to 35% in 26 months follow up. Treatment techniques also varied in device used, utilization of diverticuloscope, and extent of the myotomy, with some authors preferring to avoid complete extension through the last fibers of the cricopharyngeus in order to avoid full thickness perforation. This strategy led to expected higher rates of recurrent symptoms, but was accepted with plans for repeat endoscopic treatment sessions in order to avoid injury.
A review of 28 patients receiving flexible endoscopic needle-knife ZD therapy compared to 30 patients receiving rigid endoscopic stapling was performed by Repici. This revealed an average shorter operative time for the flexible endoscopic group (43 minutes) versus rigid stapling (63 minutes). Similar relief of dysphagia, complication rates and length of stay were found between the two groups. Relief of dysphagia was similar between the two groups. Two of the flexible endoscopy group and three of the rigid stapling group developed recurrent symptoms, all of which were treated with flexible endoscopic revision successfully.
Recently Halland et al. published their experience of 52 patients receiving flexible endoscopic treatment for ZD. They experienced higher rates of both complication and symptom recurrence. After initial improvement of dysphagia, at median 21 months the majority were symptom free, but 24% had recurrent symptoms and were retreated endoscopically, after which 12% remained symptomatic. The rate of adverse events was 28% including micro-perforations of 16%, though only 4% of adverse events (perforations requiring endoscopic stenting or drainage of neck abscess) were clinically significant. Of this group 23% were revisions who had prior rigid or endoscopic treatment, and 12% were referred for flexible endoscopy who were deemed non-surgical candidates. The authors noted a shift in the rate of complications from the first half to the second half of their experience. The risk of moderate or severe adverse events decreased with experience P=0.03. In the first 26 cases overall adverse events was 28% compared to 15% in the last 33 cases. While not reaching statistical significance (P=0.24), this was felt to be of clinical significance.
Costamagna reviewed a large series of flexible endoscopic cases to identify prognostic indicators for symptomatic recurrence. Overall recurrence in this group was significant, with clinical success of 69%, 64%, and 46% at 6, 24, and 48 months respectively at intention-to-treat analysis. Independent variables for failure to achieve symptom relief were septotomy length of 25 mm or less, and pretreatment ZD size 50 mm or greater, and at 48 months residual pouch size of 10 mm or greater. For ZD between 30 and 49 mm with a septotomy of at least 25 mm, the success rate of treatment was 100% and 71% at 6 months and 48 months, respectively (29).
Conclusions
The level of evidence for superiority of rigid versus flexible endoscopic techniques for treatment of ZD is limited based on currently available information. Data for this topic is limited, heterogeneous, and based on retrospective observations and case-control studies. Lack of objective follow up is a deficiency of almost all reviews. The groups are difficult to compare and there is so far no consensus recommendation.
Rigid endoscopic stapling is feasible for moderate to large diverticula, whereas with smaller diverticula but the design of staplers makes it difficult to complete the last 1 cm or so of the myotomy. Other dissection devices in addition to the endoscopic stapler may be required to complete the end of the myotomy. This technique requires specialized endoscopic equipment, and familiarity with its use to avoid injury. Even so, the technique is relatively standardized, which may shorten the learning curve. Stapled techniques perhaps have a slight advantage regarding risk micro-perforation or emphysema compared to flexible endoscopy.
Flexible endoscopic therapy for ZD is a good choice, particularly for select patients. Ideal indications include patients with contraindications for open or rigid transoral approaches, those with small diverticula, and failure of other endoscopic or surgical ZD treatments. The flexible endoscopic approach is acceptable alternative for all but the most giant diverticula and has become our approach of choice.
For flexible endoscopy, the rate of recurrent dysphagia is significant but highly variable across studies. Recurrence with time ranges from about 10–30%, but perhaps as high as >50% with longer follow up. Need for revision endoscopy or surgery may vary depending on the degree of recurrent dysphagia and aggressiveness of the surgeon or endoscopist (28,30). Since safely repeated, this still may be the better strategy for this chronic disease (30).
Perforation is not uncommon with any approach but is perhaps slightly higher in the flex endo approach, but reportedly few have been clinically significant. Micro-perforation of with temporary emphysema of CO2, which is usually of no clinical consequence, seems unfair to group this in perforations. Mediastinitis or abscess or require prolonged hospital stays and re-interventions are quite uncommon. It seems that this may be comparable to the POEM experience, in which exposing the submucosal layer to endoscopic insufflation often leads to tracking into the mediastinum or peritoneum, but this rarely has sequela of leak/infection or significant discomfort as long as CO2 is used for insufflation. Clinically significant leaks can usually be managed conservatively, or endoscopically with clip or stent placement (13,29).
In our center, all patients with ZD are offered flexible endoscopic treatment with few exceptions. We find that despite a slightly higher rate of recurrent symptoms, many patients are more accepting of repeat endoscopic therapy in order to avoid an open neck operation. Over the last few years we have adopted NOTE/POEM techniques for a more aggressive extended myotomy and have seen a dramatic decrease in recurrent dysphagia. A benefit may also exist for the surgical or interventional endoscopist, as this technique is that it does not require a specialized endoscope, and the dissection techniques are similar to those utilized in endoscopic submucosal dissection. Perhaps this may improve the learning curve for specialists familiar with that procedure.
The final conclusion is that all approaches to Zenkers have a place and an individualized approach should be utilized. Patient anatomical characteristics, comorbidities, procedural history, and experience of the surgeon/endoscopist should guide selection of technique. Any treatment for ZD should be performed by an expert to achieve low complication rates and safely manage complications that do occur.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Stewart K, Sen P. Pharyngeal pouch management: an historical review. J Laryngol Otol 2016;130:116-20. [Crossref] [PubMed]
- Laccourreye O, Ménard M, Cauchois R, et al. Esophageal diverticulum: diverticulopexy versus diverticulectomy. Laryngoscope 1994;104:889-92. [Crossref] [PubMed]
- Sen P, Lowe DA, Farnan T. Surgical interventions for pharyngeal pouch. Cochrane Database Syst Rev 2005.CD004459. [PubMed]
- Payne WS. The treatment of pharyngoesophageal diverticulum: the simple and complex. Hepatogastroenterology 1992;39:109-14. [PubMed]
- DeMeester T, Bremner CG. Selective cricopharyngeal myotomy for Zenker's diverticulum. J Am Coll Surg 2003;196:451-2. [Crossref] [PubMed]
- Aly A, Devitt PG, Jamieson GG. Evolution of surgical treatment for pharyngeal pouch. Br J Surg 2004;91:657-64. [Crossref] [PubMed]
- Yuan Y, Zhao YF, Hu Y, et al. Surgical treatment of Zenker's diverticulum. Dig Surg 2013;30:207-18. [Crossref] [PubMed]
- Bonavina L, Bona D, Abraham M, et al. Long-term results of endosurgical and open surgical approach for Zenker diverticulum. World J Gastroenterol 2007;13:2586-9. [Crossref] [PubMed]
- Bradley PJ, Kochaar A, Quraishi MS. Pharyngeal pouch carcinoma: real or imaginary risks? Ann Otol Rhinol Laryngol 1999;108:1027-32. [Crossref] [PubMed]
- Dohlman G, Mattsson O. The endoscopic operation for hypopharyngeal diverticula: a roentgencinematographic study. AMA Arch Otolaryngol 1960;71:744-52. [Crossref] [PubMed]
- van Overbeek JJ, Hoeksema PE, Edens ET. Microendoscopic surgery of the hypopharyngeal diverticulum using electrocoagulation or carbon dioxide laser. Ann Otol Rhinol Laryngol 1984;93:34-6. [Crossref] [PubMed]
- Collard JM, Otte JB, Kestens PJ. Endoscopic stapling technique of esophagodiverticulostomy for Zenker's diverticulum. Ann Thorac Surg 1993;56:573-6. [Crossref] [PubMed]
- Halland M, Grooteman KV, Baron TH. Flexible endosopic management of Zenker's diverticulum: characteristics and outcomes of 52 cases at a tertiary referral center. Dis Esophagus 2016;29:273-7. [Crossref] [PubMed]
- Moreira da Silva BA, Germade A, Pérez Citores L, et al. Endoscopic diverticulotomy using Ligasure™. Gastroenterol Hepatol 2017;40:80-4. [Crossref] [PubMed]
- Hondo FY, Maluf-Filho F, Giordano-Nappi JH, et al. Endoscopic treatment of Zenker's diverticulum by harmonic scalpel. Gastrointest Endosc 2011;74:666-71. [Crossref] [PubMed]
- van Overbeek JJ. Meditation on the pathogenesis of hypopharyngeal (Zenker's) diverticulum and a report of endoscopic treatment in 545 patients. Ann Otol Rhinol Laryngol 1994;103:178-85. [Crossref] [PubMed]
- Roth JA, Sigston E, Vallance N. Endoscopic stapling of pharyngeal pouch: a 10-year review of single versus multiple staple rows. Otolaryngol Head Neck Surg 2009;140:245-9. [Crossref] [PubMed]
- Bonavina L, Aiolfi A, Scolari F, et al. Long-term outcome and quality of life after transoral stapling for Zenker diverticulum. World J Gastroenterol 2015;21:1167-72. [Crossref] [PubMed]
- Hondo FY, Giordano-Nappi JH, Pessorrusso FC, et al. Comparison of electrical current and ultrasonic device for incision of the septum of the pharyngoesophageal diverticulum in a pig model. Surg Endosc 2015;29:3409-13. [Crossref] [PubMed]
- Nielsen HU, Trolle W, Rubek N, et al. New technique using LigaSure for endoscopic mucomyotomy of Zenker's diverticulum: diverticulotomy made easier. Laryngoscope 2014;124:2039-42. [Crossref] [PubMed]
- Sakai P. Endoscopic myotomy of Zenker's diverticulum: lessons from 3 decades of experience. Gastrointest Endosc 2016;83:774-5. [Crossref] [PubMed]
- Ishioka S, Sakai P, Maluf Filho F, et al. Endoscopic incision of Zenker's diverticula. Endoscopy 1995;27:433-7. [Crossref] [PubMed]
- Mulder CJ, den Hartog G, Robijn RJ, et al. Flexible endoscopic treatment of Zenker's diverticulum: a new approach. Endoscopy 1995;27:438-42. [Crossref] [PubMed]
- Rieder E, Martinec DV, Dunst CM, et al. Flexible endoscopic Zenkers diverticulotomy with a novel bipolar forceps: a pilot study and comparison with needleknife dissection. Surg Endosc 2011;25:3273-8. [Crossref] [PubMed]
- Rabenstein T, May A, Michel J, et al. Argon plasma coagulation for flexible endoscopic Zenker's diverticulotomy. Endoscopy 2007;39:141-5. [Crossref] [PubMed]
- Christiaens P, De Roock W, Van Olmen A, et al. Treatment of Zenker's diverticulum through a flexible endoscope with a transparent oblique-end hood attached to the tip and a monopolar forceps. Endoscopy 2007;39:137-40. [Crossref] [PubMed]
- Case DJ, Baron TH. Flexible endoscopic management of Zenker diverticulum: the Mayo Clinic experience. Mayo Clin Proc 2010;85:719-22. [Crossref] [PubMed]
- Pescarus R, Shlomovitz E, Sharata AM, et al. Trans-oral cricomyotomy using a flexible endoscope: technique and clinical outcomes. Surg Endosc 2016;30:1784-9. [Crossref] [PubMed]
- Costamagna G, Iacopini F, Bizzotto A, et al. Prognostic variables for the clinical success of flexible endoscopic septotomy of Zenker's diverticulum. Gastrointest Endosc 2016;83:765-73. [Crossref] [PubMed]
- Brueckner J, Schneider A, Messmann H, et al. Long-term symptomatic control of Zenker diverticulum by flexible endoscopic mucomyotomy with the hook knife and predisposing factors for clinical recurrence. Scand J Gastroenterol 2016;51:666-71. [Crossref] [PubMed]