Optical imaging of tissue obtained by transbronchial biopsies of peripheral lung lesions
Introduction
Tissue biopsy sampling is essential for an accurate diagnosis of lung cancer (1). There are several means of diagnosing peripheral lung lesions, including transthoracic image-guided procedures, bronchoscopic procedures, and surgery. The results of the AQuiRe data registry for the diagnostic yield and complications of bronchoscopy for peripheral lung lesions were recently published by Ost et al. (2). Both navigational bronchoscopy and radial endobronchial ultrasound (R-EBUS) performed poorly, with a yield of 57% and 38.5% respectively and 47.1% when the two modalities were combined. Optical spectroscopy can provide vascular and biochemical contrasts, which can produce enhanced sensitivity and specificity for tissue characterization. This was demonstrated in prior studies in oral, oropharyngeal, and lung cancers (3-12). The primary objective of this study is to assess the feasibility and ability of a custom-built bimodal optical spectroscopy system combining diffuse reflectance spectroscopy (DRS) and diffuse fluorescence spectroscopy (DFS) to enhance the on-site discrimination between malignant and benign specimens that are obtained by transbronchial biopsies of peripheral lung lesions.
Methods
This study is a single-centered prospective pilot trial examining the lung tissue samples obtained through transbronchial biopsy. The study entails using the bimodal optical spectroscopy system to quantify multiple physiologic parameters of interest. The sensitivity and specificity of these parameters to differentiate between malignant and benign transbronchial lung biopsies (TBLB) specimens was investigated. The study obtained ethical approval from Roswell Park Cancer Institute Institutional Review Board (I-246913). All enrolled subjects gave informed consent before taking part in this study.
Ex-vivo specimens’ collection
Lung tissues were obtained from 15 patients using TBLB for the diagnosis of peripheral lung lesions. The flexible bronchoscopy procedures combined with R-EBUS and navigation system were used in all cases. All the procedures were performed under general anesthesia in the endoscopy suite.
Instrumentation
The instrumentation consisted of a custom-built, fiber-based, two-channel spectroscopy system, where one channel allowed white light reflectance measurements for quantification of optical and hemoglobin-related parameters, and the other channel allowed fluorescence measurements (Figure 1). The white light reflectance fiber was connected to a tungsten halogen light source (HL-2000-FHSA, Ocean Optics) and the fluorescence fiber was connected to a 365 nm LED (M365F1, ThorLabs) for fluorescence excitation. The white light and fluorescence detector fibers were connected to the less-sensitive (Master) and more sensitive (Slave) channels respectively of a two-channel spectrometer (SD2000, Ocean Optics). The source-detector separations for both channels were 520 µm. Measurements were acquired by rinsing the biopsy sample with saline to remove any residual blood from the sample and placing it under a blacked-out container to minimize the effect of room light. The probe tip (1 mm diameter) made soft contact with the sample surface. Multiple measurements (n>5) were acquired by slightly repositioning the probe on each sample. After measurements were completed, a reference standard was measured for the calibration of the instrument.
Optical measurements
For each spectroscopic measurement, the background counts were subtracted, and the signal was normalized with the reflectance standard to obtain the normalized reflectance. The normalized reflectance was scaled to the diffusion model in order to obtain reflectance in absolute units (13). This step is needed for accurate quantification due to the short separation between the source and detector fiber. To determine the scale factor for the probe, we followed a similar calibration procedure to that described in (13).
To extract the blood and scattering-related parameters, a trust-region-reflective nonlinear fitting algorithm (lsqnonlin, MATLAB) was used to fit the scaled data with the diffusion model. Tissue absorption was expressed in terms of blood volume (BV), tissue oxygen saturation (StO2), and a water fraction (fixed at 0.65) (5,6). BV and StO2 could also be expressed as the concentrations of oxygenated and deoxygenated hemoglobin [(HbO2) and (Hb), respectively]. The reduced scattering coefficient (µs’) was modeled as Mie scattering, µs’(λ) = A(λ/λ0)-b, where λ0 =800 nm, A characterizes the magnitude of scattering and b characterizes the wavelength dependence (14). For fluorescence measurements, background counts were subtracted, and the spectra were normalized by the reflected excitation light that “leaked” through the long pass filter. This partially corrected for the effect of optical properties at the excitation wavelength. Fluorescence values are reported as the magnitude of the normalized spectra in the green fluorescence band (495 to 530 nm) and the red fluorescence band (585 to 620 nm).
Histopathological evaluation
After optical measurements, each biopsy specimen was placed in separately labeled containers with formalin 10% solution. All specimens were sent for routine histopathology analysis. The individual performing the measurements was blinded to the histopathologic diagnosis, and the pathologist was blinded to the optical measurements.
Statistical analysis
The blood, scattering, and fluorescence parameters were reported by pathological diagnosis using the mean and standard error (Table 1). Comparisons were made using a linear mixed model, which accounts for the multiple observations per patient. Model assumptions were verified graphically and Box-Cox transformations were applied as appropriate.
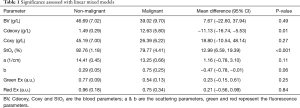
Full table
To determine the utility of optical imaging parameters for cancer classification, the four non-cancer groups (normal, inflammation, blood clot and necrotic debris) were combined and compared against the cancer group. A secondary analysis was performed for the cancer versus necrotic debris groups. Multiple parameters were combined into a single classifier using generalized estimating equations (GEE) logistic regression; where only combinations of three parameters (one blood, scattering and fluorescence) were tested to avoid overfitting. Receiver operating characteristic (ROC) curves and corresponding area under the curve (AUC) results were obtained for each parameter with respect to cancer vs. non-cancer and cancer vs. necrotic. The sensitivity and specificity were determined at the Youden Index and the positive predictive value (PPV) and negative predictive value (NPV) were determined using the group sizes to estimate prevalence.
All analyses were conducted in SAS v 9.4 (Cary, NC) at a significance level of 0.05; therefore a P-value less than 0.05 is considered statistically significant. As a pilot study, no adjustments were made for multiple testing.
Results
Sixteen patients were enrolled. One patient was excluded after obtaining the diagnosis from a hilar lymph node and no transbronchial biopsies were performed. There was a total of n=116 unique biopsy specimens with a confirmed pathologic diagnosis of 15 patients [20% male and an average age of 64.2 (SD =9.9; range 43–82)]. Twenty-two of the 116 specimens were malignant, and 10 of the 94 non-malignant specimens were necrotic biopsies. Fluorescence measurements were not acquired for 16 non-malignant biopsies. The necrotic specimens were all from one patient. Seventeen of the 22 malignant specimens were lung cancers and 5 were colon cancers. The benign specimens were reported as acute or chronic inflammatory, fibrotic or cartilage, blood clots, bronchial or parenchymal tissues.
The blood parameters Cdeoxy (concentration of deoxygenated hemoglobin) and StO2 (saturation of oxygen) were found significant in differentiating malignant from benign specimens. The malignant specimens had higher Cdeoxy and lower StO2 compared to benign specimens (12.63 vs. 1.49 g/L and 79.7% vs. 92.76%, respectively). All other imaging parameters were not statistically significant (Table 1).
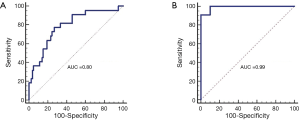
The best combination, based on the AUC, of three parameters for classifying malignant vs. benign and malignant vs. necrotic was the concentration of deoxy- hemoglobin, the scattering parameter b and the red fluorescence intensity. The sensitivity and specificity for differentiating between malignant and benign specimens was 77.3% and 73.1%, respectively with an AUC of 0.80 (Figure 2A). The resulting positive and NPVs were 44.7% and 91.9%, respectively with 17 true positives, 21 false positives, 57 true negatives and 5 false negatives. The sensitivity and specificity for differentiate between malignant and necrotic specimens were 90.9% and 100.0%, respectively (Figure 2B) with an AUC of 0.99. The resulting positive and NPVs were 100.0% and 83.3%, respectively with 20 true positives, 0 false positives, 10 true negatives and 2 false negatives.
Discussion
Our study demonstrates that the combination of DRS and DFS exhibited good sensitivity and specificity for on-site discrimination between malignant and benign TBLB specimens during the bronchoscopy procedures. It also showed high sensitivity and specificity in discriminating between necrotic and malignant TBLB specimens.
This is a pilot study, and the primary aim was to evaluate the feasibility of this technique. The high sensitivity of the described imaging modality to discriminate between malignant and benign is of significant importance. It means that the proceduralist can have a high degree of confidence that the TBLB specimen is malignant and may, therefore, decrease the number of biopsies needed and potentially lowering the complication rate associated with lung biopsies such as bleeding and pneumothorax. Our study showed that optical spectroscopy measurements of TBLB were highly sensitive and specific for differentiating between necrotic and malignant specimens but given that all necrotic specimens were from a single patient, these findings are secondary and need further investigation in larger trials. This is essential as many tumors are necrotic and the proceduralist may face a situation where the tumor is appropriately sampled, but the final histopathologic examination is mostly necrotic and therefore nondiagnostic. Our imaging technique would allow the proceduralist to change or adjust the location from where the TBLB are obtained and therefore manipulate the biopsy forceps to get TBLB specimen from a more malignant rather than necrotic area of the targeted lesion. Given that our main objective was to evaluate the feasibility of the optical spectroscopy for the diagnosis of TBLB, a larger prospective study is warranted to evaluate the effectiveness of this technique and to identify factors associated with outcome such as the size of specimens and the ratio of tumor cell to non-tumor cells in the specimens.
The optical measurements were performed directly after obtaining the specimens from transbronchial biopsies and before fixing the specimens in formalin. This is advantageous as it offers the rapid on-site evaluation of the specimens obtained and immediate perspective regarding the direction of the diagnosis. Optical spectroscopy has been shown to be effective in characterizing cancer tissue in oral cavity and lung (3-5,7,8,10,11). In a recent study by Spliethoff et al., the lung tissues obtained from lobectomy or segmental lung resections from 13 patients were imaged using optical spectroscopy. The tumor size ranged between 8 and 50 mm. DRS and DFS were highly successful in discriminating between malignant and normal lung tissue with sensitivity and specificity of 98% and 86% respectively, and between necrotic and non-necrotic tumor with sensitivity and specificity of 91% (3). In a recent study per Spliethoff et al., transthoracic application of DRS was used in 21 patients intraoperatively during surgery for lung resection and in 11 patients undergoing routine transthoracic lung biopsy for peripheral lung lesions. DRS was able to differentiate between malignant and surrounding tissue with high sensitivity and specificity. The positive findings were attributed to the water content DRS parameters and not the blood parameters.
As Tromberg group and others indicated tissue hemoglobin oxygen saturation levels (StO2) at the tumor are lower than those in surrounding normal areas, due to local metabolic activity: tumor cells are more metabolically active and consumes more oxygen, thus overall StO2 levels are lower in tumor tissue, compared to surrounding normal tissue (15,16). Deoxy-hemoglobin (Cdeoxy) may be a more sensitive index of oxygen consumption and local metabolism. If the oxygen is consumed in tumor, then the deoxy-hemoglobin levels increases. Thus, we can say that while oxy-hemoglobin changes can be attributed primarily to vascular oxygen supply, deoxy-hemoglobin concentration is representative of tumor tissue oxygen consumption. As tumor cells undergo apoptosis and reduce proliferation, oxygen consumption may diminish.
On-site discrimination between malignant and benign biopsies remains a significant challenge for bronchoscopists. The outcome of transbronchial biopsies is reported as a diagnostic or non-diagnostic specimen, or diagnostic but not sufficient specimen for further analysis such, Immunohistochemistry (IHC), molecular or genetic testing. Rapid-on-site evaluation (ROSE) can be used for TBNA and transbronchial brush for intraoperative cytological assessment. However, ROSE is not universally available and does not allow for histologic evaluation of the TBLB (17). Squash smear cytology has been shown to be helpful when the TBLB specimens are very small or in cases where only a few specimens are available (18). This method has not been used for normal-size TBLB specimens (2). Frozen section of the TBLB specimens is not a common practice, and this is due to the high cost, limited availability, and the inability of using the frozen specimen for further specimen identification using pathology stains, molecular testing or others (2,19). Coghlin et al. demonstrated that specimens from bronchial biopsies frequently contain limited amounts of malignant cells, and in only 48% of cases, tumor was found in all biopsy specimens (20). Furthermore, the bronchoscopists may not be able to assess the quality of TBLB accurately. This was shown in a study by Curley et al. where the physician’s rating of the specimens’ quality did not vary between specimens containing normal or abnormal tissue or between diagnostic and nondiagnostic tissue (19). The efficacy of the optical spectroscopy approach needs to be assessed on a larger scale and with a wider range of lung pathology to establish it as a standard of care.
The ex vivo nature of the optical measurement of TBLB specimens in this study may be considered a limitation. However, the advantage of an ex vivo analysis of TBLB is the simplicity of the technique (outside this pilot study) and the no need for the proceduralist to learn a new technology that may require more training and advanced level of expertise. The future direction will be to investigate the in vivo optical imaging by building a catheter that can be used bronchoscopically to be directed to the peripheral lung lesions. The in vivo optical measurement may offer more information about the hemodynamic characteristics of the targeted lesion before obtaining the TBLB and therefore may effectively guide the proceduralist. The in vivo optical imaging can be challenging as blood during TBLB may alter the measurement and interpretation of the results. Additionally, the in vivo imaging will probably require a significant training and advanced level of expertise, which could limit the popular use of such technique.
This study has some other limitations, most importantly the technical challenges in obtaining the optical measurements given that this is a pilot study and we were testing the feasibility of this technique. The optical measurements were performed manually and required an individual with expertise. Building an automated system to perform the optical measurements will overcome these technical challenges and will make optical measurements of TBLB specimens instant and simple with no need for specific training or assistance. Another limitation is that each measurement was point-based, simply providing the bulk property of each biopsy sample. In the future high-resolution imaging based system would allow maps of the samples and assess the heterogeneity in the contrasts.
Conclusions
Optical spectroscopy of the TBLB specimens is feasible, and moderately sensitive and specific in discriminating malignant from benign pathology and highly sensitive and specific for differentiating necrotic from tumor-containing specimens. This is a pilot study and larger prospective trials are needed to further evaluate the effectiveness of this technique for TBLB.
Acknowledgements
Funding: This work was supported by National Cancer Institute (NCI) grant P30CA016056 and by a grant from the Ohio Third Frontier to the Ohio Imaging Research and Innovation Network (OIRAIN).
Footnote
Conflicts of Interest: K Harris is a consultant for Cook Medical. The other authors have no conflicts of interest to declare.
Ethical Statement: The study obtained ethical approval from Roswell Park Cancer Institute Institutional Review Board (I-246913). All enrolled subjects gave informed consent before taking part in this study.
References
- National Lung Screening Trial Research Team., Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Ost DE, Ernst A, Lei X, et al. Diagnostic Yield and Complications of Bronchoscopy for Peripheral Lung Lesions. Results of the AQuIRE Registry. Am J Respir Crit Care Med 2016;193:68-77. [Crossref] [PubMed]
- Spliethoff JW, Evers DJ, Klomp HM, et al. Improved identification of peripheral lung tumors by using diffuse reflectance and fluorescence spectroscopy. Lung Cancer 2013;80:165-71. [Crossref] [PubMed]
- Spliethoff JW, Prevoo W, Meier MA, et al. Real-time In Vivo Tissue Characterization with Diffuse Reflectance Spectroscopy during Transthoracic Lung Biopsy: A Clinical Feasibility Study. Clin Cancer Res 2016;22:357-65. [Crossref] [PubMed]
- Rohrbach DJ, Rigual N, Tracy E, et al. Interlesion differences in the local photodynamic therapy response of oral cavity lesions assessed by diffuse optical spectroscopies. Biomed Opt Express 2012;3:2142-53. [Crossref] [PubMed]
- Sunar U, Rohrbach D, Rigual N, et al. Monitoring photobleaching and hemodynamic responses to HPPH-mediated photodynamic therapy of head and neck cancer: a case report. Opt Express 2010;18:14969-78. [Crossref] [PubMed]
- Rohrbach DJ, Rigual N, Arshad H, et al. Intraoperative optical assessment of photodynamic therapy response of superficial oral squamous cell carcinoma. J Biomed Opt 2016;21:18002. [Crossref] [PubMed]
- Amelink A, Kaspers OP, Sterenborg HJ, et al. Non-invasive measurement of the morphology and physiology of oral mucosa by use of optical spectroscopy. Oral Oncol 2008;44:65-71. [Crossref] [PubMed]
- Bard MP, Amelink A, Hegt VN, et al. Measurement of hypoxia-related parameters in bronchial mucosa by use of optical spectroscopy. Am J Respir Crit Care Med 2005;171:1178-84. [Crossref] [PubMed]
- Bard MP, Amelink A, Skurichina M, et al. Improving the specificity of fluorescence bronchoscopy for the analysis of neoplastic lesions of the bronchial tree by combination with optical spectroscopy: preliminary communication. Lung Cancer 2005;47:41-7. [Crossref] [PubMed]
- van der Leest C, Amelink A, van Klaveren RJ, et al. Optical detection of preneoplastic lesions of the central airways. ISRN Oncol 2012;2012:957835. [Crossref] [PubMed]
- Gallagher-Colombo SM, Quon H, Malloy KM, et al. Measuring the Physiologic Properties of Oral Lesions Receiving Fractionated Photodynamic Therapy. Photochem Photobiol 2015;91:1210-8. [Crossref] [PubMed]
- Kim A, Roy M, Dadani F, et al. A fiberoptic reflectance probe with multiple source-collector separations to increase the dynamic range of derived tissue optical absorption and scattering coefficients. Opt Express 2010;18:5580-94. [Crossref] [PubMed]
- Mourant JR, Fuselier T, Boyer J, et al. Predictions and measurements of scattering and absorption over broad wavelength ranges in tissue phantoms. Appl Opt 1997;36:949-57. [Crossref] [PubMed]
- Boas DA, Franceschini MA. Haemoglobin oxygen saturation as a biomarker: the problem and a solution. Philos Trans A Math Phys Eng Sci 2011;369:4407-24. [PubMed]
- Cerussi A, Hsiang D, Shah N, et al. Predicting response to breast cancer neoadjuvant chemotherapy using diffuse optical spectroscopy. Proc Natl Acad Sci U S A 2007;104:4014-9. [Crossref] [PubMed]
- Trisolini R, Cancellieri A, Tinelli C, et al. Randomized Trial of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration With and Without Rapid On-site Evaluation for Lung Cancer Genotyping. Chest 2015;148:1430-7. [Crossref] [PubMed]
- Nayanar SK, Puthiyaveettil AK, Bhasurangan KC. Utility of squash smear cytology in fiber-optic bronchoscopic biopsies. J Cytol 2014;31:11-4. [Crossref] [PubMed]
- Curley FJ, Johal JS, Burke ME, et al. Transbronchial lung biopsy: can specimen quality be predicted at the time of biopsy? Chest 1998;113:1037-41. [Crossref] [PubMed]
- Coghlin CL, Smith LJ, Bakar S, et al. Quantitative analysis of tumor in bronchial biopsy specimens. J Thorac Oncol 2010;5:448-52. [Crossref] [PubMed]



