Plasma levels of matrix metalloproteinase 9 in patients undergoing off-pump coronary artery bypass grafting
Introduction
Matrix metalloproteinases (MMPs) are zinc-binding endopeptidases with multifactorial actions in the outcome of various pathological and physiological processes including atherosclerosis and congestive heart failure (1,2). In the physiological condition, structure and function of the extracellular matrix are maintained by the MMPs family (3). Importantly, in pathophysiological conditions changes of ischemia in activity of the MMP enzymes are selective. The major causes of cardiac ischemia are due to low blood flow, hypothermia, hypotension, inflammatory response, and atheroembolism, which may be responsible for ischemia-reperfusion injuries (4). These factors exist widely in the pathophysiology of perioperative myocardial injury. Early detection of perioperative biomarkers may play an important role in the timely diagnosis and treatment of perioperative myocardial injury.
The gelatinase MMP9 is specifically implicated in adverse left ventricular remodeling and involved in acute processes such as vascular tone regulation and the aggregation of platelets (5-7). In a former clinical study, brain natriuretic peptide (BNP) decreases collagen synthesis and overexpression increases MMPs activity (8). In addition, plasma MMP9 increases significantly at the early period after myocardial infarction (9,10). Myocardial injury is a common complication occurring during the perioperative period, and is related with adverse outcomes such as higher mortality (11). In response to ischemia reperfusion (I/R) as well as inflammation and oxidative stress, expression levels of MMP9 rise significantly (12). This biomarker in plasma seems particularly relevant in the coronary artery bypass grafts surgery procedure, in which most of the above mechanisms are involved.
Off-pump coronary artery bypass grafting (OPCAB) is one of the standard procedures for surgical revascularization in patients with coronary artery disease (CAD), especially in Asia. However, to our knowledge, no adequate analyzed data exist about the potential interest of role and impact of MMP9 in the occurrence of myocardial injury during OPCAB procedure. We therefore designed the present study to evaluate plasma levels of MMP9 and their diagnostic validity in patients undergoing OPCAB, and to assess its relations to the common biomarkers of myocardial injury and clinical cardiac dysfunction.
Methods
Patients population
A prospective observational study was conducted in this research. During the period from June to October 2015, 34 consecutive patients in total participated in this study at the Department of Cardiac Surgery, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China. Patients were excluded if they were matched with one or more of the criteria as follows: age <18 or >80 years, emergency operation, having suffered primary and secondary myocardiopathy, with the report of the acute coronary syndrome within 30 days, presence of infectious or malignant tumor, suffering inflammatory disease or autoimmune disease, patients treated with corticosteroids.
Perioperative management
In the operating room, after a radial artery hemodynamic monitoring system was set up, anesthesia was induced with intravenous midazolam (0.03 mg/kg), propofol (2.0 mg/kg), and sufentanil (0.5 mg/kg/h). Patients were intubated and ventilated with FiO2: 40%. Following median sternotomy, the saphenous vein graft and left internal mammary artery (LIMA) were harvested. Off-pump procedure was performed on the heart utilizing an intracoronary shunt (Guidant Axius Coronary Shunt, Guidant Corporation, Santa Clara, CA, USA) and a stabilizer (Octopus Medtronic, MN, USA). A side-biting aorta clamp was used when proximal anastomoses were performed. Flow measurements are carried out with transit time flow probes (Medtronic, Inc., USA). Patients were transferred to the postoperative intensive care unit (ICU) after closure of the sternum.
Clinical data collection
On the basis of medical records, the collection of enrolled patients’ following clinical variables data was comprehensive, such as age, sex, usual cardiovascular risk factors, cardiovascular and pulmonary diseases, regular medication in the past, and the parameters of transthoracic echocardiography. Investors made assessment of the Echocardiographic data sets under the condition of not knowing the result of laboratory.
Blood sampling and biochemical analyses
On the morning of the second day after being admitted to hospital (before the surgery), enrolled patients’ blood samples were collected and analyzed. The blood samples played the role of the baseline reference values. Many measurements were proceeded, such as: C-reactive protein (CRP), N-terminal pro B-type natriuretic peptide (NT-proBNP), creatinine kinase myocardial b fraction (CK-MB), cardiac troponin I (cTnI), low-density lipoprotein cholesterol and creatinine. All biochemical analyses were performed by investigators blinded to the clinical data of the patients.
Preoperative (pre-OP) time point and 12 hours after being sent to the ICU, the two points were used to measure the level of plasma MMP9 of all the patients. Blood collected from patients with the volume of 3 mL was stored in a heparin anticoagulant tube. After incubation at room temperature for 2 h, blood samples were centrifuged and the plasma was immediately stored at –80 °C until measurement. Plasma MMP9 concentrations were measured in duplicate by quantitative sandwich enzyme immunoassay (Sigma-Aldrich, St Louis, MO, USA).
Statistical analyses
Data were analyzed using statistical software SPSS version 19.0 (SPSS Inc., Chicago, Illinois, USA) and GraphPad Prism 6.0 (GraphPad Software Inc., California, USA). Continuous variables are presented as means ± standard error of mean (SEM) unless otherwise indicated; categorical variables as numbers (percentages). Normality was checked by the Kolmogorov-Smirnov test for continuous data, nonparametric variables were reported as medians with interquartile ranges (IQR). Differences between unpaired groups were analyzed using a Mann-Whitney U test for continuous variables. A double-sided P value <0.05 was considered statistically significant for all tests. Associations between MMP9 and laboratory parameters were tested by Spearman’s correlation rank test.
Results
Basic clinical characteristics of patients
The pre-OP clinical and laboratory information of the 34 patients enrolled in the study are listed in Table 1. The mean age was 65.1±1.7 years and males constituted 79.4% of the total subjects. Factors, such as hypertension, diabetes mellitus, dyslipidemia and smoking were 61.8%, 38.2%, 26.5% and 44.1% respectively. Creatinine were 90.8±5.8 (range, 56.0–250.0) mmol/L, cTnI were 0.01 (range, 0.01–1.78) ng/mL, CRP were 0.50 (range, 0.18–1.28) mg/L (range, 0.10–7.00 mg/L), and NT-proBNP levels were 537.4±103.7 (range, 5.0–2,036.0) ng/L. All patients adopted off-pump cardiac surgery. Pre-OP left ventricular ejection fraction (LVEF) (%) was 63.1±1.3 and the mean European System for Cardiac Operative Risk Evaluation (EuroSCORE) II was 2.47.
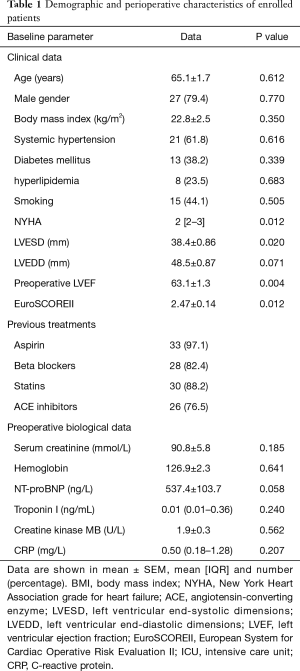
Full table
Relationship between plasma pre-OP MMP9 concentrations and baseline characteristics
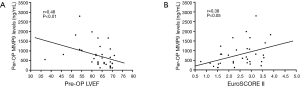
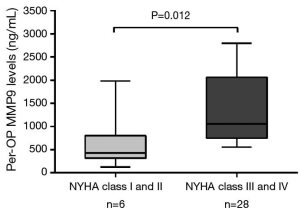
Mean MMP9 levels were 786.4±106.6 (range, 120.3–2,796.0) ng/L. Associations between laboratory parameters and MMP9 plasma concentrations were tested using Spearman’s correlation rank test. There was a significant correlation between pre-operative (pre-OP) circulating levels of MMP9 and the LVEF (r=0.48; P=0.004, Figure 1A) as well as EuroSCOREII (r=0.43; P=0.012, Figure 1B). In contrast, no significant correlation was observed between MMP9 and age (P=0.612), serum creatinine (P=0.185), CRP (P=0.207), NT-proBNP (P=0.058), and cTnI (P=0.240). Moreover, regarding categorical variables, MMP9 levels were insignificantly related to the hypertension (799.5±138.4 vs. 688.4±167.0 ng/L, P=0.48) and diabetes (908.4±210.1 vs. 663.3±110.5 ng/L, P=0.34), hyperlipemia (695.8±205.4 vs. 775.9±124.6 ng/L, P=0.68). At enrollment, 6 of 34 patients were in New York Heart Association (NYHA) functional class III or IV heart failure and showed a significantly higher MMP9 levels (1,348.0±337.2 vs. 630.4±93.0 ng/L, P=0.012) as compared to the patients in NYHA functional class I and II (Figure 2).
Relationship between levels of post-OP MMP9 and myocardial injury
The postoperative laboratory and clinical data of all the patients in the study are listed in Table 2. The circulating levels of MMP9 were highly increased at 12 h after surgery in OPCAB patients as indicated by the fold change values. Mean postoperative 12 h MMP9 levels were 1,464±154.8 (range, 183.8–4,870.0) ng/L. The 12 h MMP9 levels were 2-fold (P<0.0001) higher than the pre-OP control level. To further document an association between levels of MMP9 and myocardial injury, we correlated cTnI, CK-MB levels with MMP9 levels. As illustrated in Figure 3, a significant correlation was observed in these data between the post-OP 12 h MMP9 and cTnI (r=0.35; P<0.05, Figure 3A). However, an association statistic between the post-OP 12 h MMP9 and CK-MB shows no significant association (R=0.28; P>0.05, Figure 3B). Thus, the release level of the cardiac-enriched MMP9 reflected the extent of cardiac injury as measured by cTnI release into circulation.
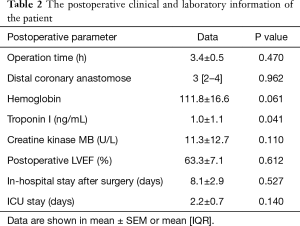
Full table

Discussion
Perioperative myocardial injury has been thought as a kind of serious complication for CABG, which is also related to a high level of morbidity and mortality during a long period (13). Under the circumstance that no effective pharmacological therapy can be used to treat the perioperative myocardial, but early preventive measures, like pre-OP risk predication and perioperative optimization, could be a good choice in reducing the myocardial injury to some degree (14). What’s more, due to a novel biomarker, myocardial injury prediction is of great use in providing the valuable information in the management during the perioperative period (15). After experiencing ischemia/reperfusion, MMP9 expression induced with a high level under the experimental conditions (16).
According to the clinical literature, some studies have stated that MMP9 has a strong correlation with cardiac injury. It has been demonstrated that MMP9 concentration’s changes could reflect both cardiovascular inflammation and other pathophysiological processes (17,18). Indeed, it is also reported that higher level of plasma MMP9 in patients who are with cardiovascular pathologies, like CAD or chronic heart failure (19-21).
For patients with acute myocardial infraction, MMP9 is a reliable biomarker when facing the fatal events (22). Furthermore, for patients with CAD, MMP9 concentration has the correlation with other inflammation biomarkers (18). Common risk factors of organ dysfunction caused by cardiac surgery have been revealed such as hypertension, diabetes, peripheral vascular disease, chronic obstructive pulmonary disease, cardiogenic shock, congestive heart failure. However, as for the particular setting of perioperative myocardial injury, and from the perspective of predict value, there is few studies can be used as reference.
The present study revealed that pre-OP MMP9 levels in patients undergoing OPCAB were significantly associated with LVEF and NYHA. This is in agreement with previous studies showing that MMP9 was a novel independent biomarker in cardiovascular disease (23,24). Apart from the pre-OP clinical markers, we also found a positively association between MMP9 and cTnI during the postoperative period. However, it was interesting that there was no substantial correlation between MMP9 and CK-MB. Persisting elevated MMP9 levels closely related to postoperative myocardial injury, revealed by the plasma cTnI levels. To our knowledge, this is the first study to demonstrate that increased MMP9 levels are significantly associated with biomarkers of postoperative cardiac injury after OPCAB.
We propose that it is valuable to measure and identify MMP9 levels combined with cardiac troponin as a more sensitive and effective biomarker than traditional cardiac troponin to diagnose perioperative myocardial injury, enabling an accurate therapeutic interventions and reducing postoperative adverse cardiac outcome.
A few limitations of this study deserve to be addressed. First, only the plasma MMP-9 concentrations and clinical parameters were analyzed during the acute phase in this study, needing further experimental data to verify the biological changes of acute cardiac injury and myocardial remodeling in future. Second, a relatively small sample size in this study may diminish its significance of correlation. More participants with various groups are essential to verify MMP-9 as a clinical indicator for cardiac dysfunction after off pump cardiac surgery.
Conclusions
A perioperative measurement of plasma MMP9 levels added a significant predictive value to the classical risk factors of cardiac surgery. MMP9 can be used as a novel biomarker combined with cTnI for the detection of perioperative myocardial injury of the OPCAB surgery in patients with CAD.
Acknowledgements
Funding: This work was supported by grants from the National Natural Science Foundation of China (81671832, 81571826 and 81200093).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Patients who were admitted to the department of cardiac surgery for treatment agreed and approved the information consent in the writing. Base on the content of Helsinki declaration, the study was approved by the independent Medical Ethics Committee of the Ruijin Hospital, Shanghai Jiaotong University School of Medicine (No. 2016095).
References
- Moore L, Fan D, Basu R, et al. Tissue inhibitor of metalloproteinases (TIMPs) in heart failure. Heart Fail Rev 2012;17:693-706. [Crossref] [PubMed]
- Wang M, Kim SH, Monticone RE, et al. Matrix metalloproteinases promote arterial remodeling in aging, hypertension, and atherosclerosis. Hypertension 2015;65:698-703. [Crossref] [PubMed]
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 2003;92:827-39. [Crossref] [PubMed]
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 2007;357:1121-35. [Crossref] [PubMed]
- Yarbrough WM, Mukherjee R, Escobar GP, et al. Selective targeting and timing of matrix metalloproteinase inhibition in post-myocardial infarction remodeling. Circulation 2003;108:1753-9. [Crossref] [PubMed]
- Fernandez-Patron C, Martinez-Cuesta MA, Salas E, et al. Differential regulation of platelet aggregation by matrix metalloproteinases-9 and -2. Thromb Haemost 1999;82:1730-5. [PubMed]
- Kelly D, Cockerill G, Ng LL, et al. Plasma matrix metalloproteinase-9 and left ventricular remodelling after acute myocardial infarction in man: a prospective cohort study. Eur Heart J 2007;28:711-8. [Crossref] [PubMed]
- Tsuruda T, Boerrigter G, Huntley BK, et al. Brain natriuretic Peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circ Res 2002;91:1127-34. [Crossref] [PubMed]
- Fukuda D, Shimada K, Tanaka A, et al. Comparison of levels of serum matrix metalloproteinase-9 in patients with acute myocardial infarction versus unstable angina pectoris versus stable angina pectoris. Am J Cardiol 2006;97:175-80. [Crossref] [PubMed]
- Šímová J, Škvor J, Slovák D, et al. Serum levels of matrix metalloproteinases 2 and 9 in patients with acute myocardial infarction. Folia Biol (Praha) 2013;59:181-7. [PubMed]
- Selvanayagam JB, Petersen SE, Francis JM, et al. Effects of off-pump versus on-pump coronary surgery on reversible and irreversible myocardial injury: a randomized trial using cardiovascular magnetic resonance imaging and biochemical markers. Circulation 2004;109:345-50. [Crossref] [PubMed]
- Kameda K, Matsunaga T, Abe N, et al. Correlation of oxidative stress with activity of matrix metalloproteinase in patients with coronary artery disease. Possible role for left ventricular remodelling. Eur Heart J 2003;24:2180-5. [Crossref] [PubMed]
- Namay D, Hammermeister KE, Zia MS, et al. Effect of perioperative myocardial infarction on late survival in patients undergoing coronary artery bypass surgery. Circulation 1982;65:1066-71. [Crossref] [PubMed]
- Parolari A, Poggio P, Myasoedova V, et al. Biomarkers in Coronary Artery Bypass Surgery: Ready for Prime Time and Outcome Prediction? Front Cardiovasc Med 2016;2:39. eCollection 2015.
- Hueb W, Gersh BJ, Alves da Costa LM, et al. Accuracy of Myocardial Biomarkers in the Diagnosis of Myocardial Infarction After Revascularization as Assessed by Cardiac Resonance: The Medicine, Angioplasty, Surgery Study V (MASS-V) Trial. Ann Thorac Surg 2016;101:2202-8. [Crossref] [PubMed]
- Johnson C, Sung HJ, Lessner SM, et al. Matrix metalloproteinase-9 is required for adequate angiogenic revascularization of ischemic tissues: potential role in capillary branching. Circ Res 2004;94:262-8. [Crossref] [PubMed]
- Halade GV, Jin YF, Lindsey ML. Matrix metalloproteinase (MMP)-9: a proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. Pharmacol Ther 2013;139:32-40. [Crossref] [PubMed]
- Ferroni P, Basili S, Martini F, et al. Serum metalloproteinase 9 levels in patients with coronary artery disease: a novel marker of inflammation. J Investig Med 2003;51:295-300. [Crossref] [PubMed]
- Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem 2008;54:24-38. [Crossref] [PubMed]
- Apple FS, Smith SW, Pearce LA, et al. Assessment of the multiple-biomarker approach for diagnosis of myocardial infarction in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chem 2009;55:93-100. [Crossref] [PubMed]
- Wagner DR, Delagardelle C, Ernens I, et al. Matrix metalloproteinase-9 is a marker of heart failure after acute myocardial infarction. J Card Fail 2006;12:66-72. [Crossref] [PubMed]
- Webb CS, Bonnema DD, Ahmed SH, et al. Specific temporal profile of matrix metalloproteinase release occurs in patients after myocardial infarction: relation to left ventricular remodeling. Circulation 2006;114:1020-7. [Crossref] [PubMed]
- Blankenberg S, Rupprecht HJ, Poirier O, et al. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation 2003;107:1579-85. [Crossref] [PubMed]
- Hansson J, Vasan RS, Ärnlöv J, et al. Biomarkers of extracellular matrix metabolism (MMP-9 and TIMP-1) and risk of stroke, myocardial infarction, and cause-specific mortality: cohort study. PLoS One 2011;6:e16185. [Crossref] [PubMed]


