Ten-year experience with endobronchial ultrasound-guided transbronchial needle aspiration: single center results in mediastinal diagnostic and staging
Introduction
Since introduction of ultrasound in bronchoscopy in 1992 (1) major technological advances have occurred. Endo-bronchial ultrasound (EBUS) has revealed useful in the assessment of the airway layer as well as in real time biopsies of lymph-nodes and central tumors in association with transbronchial needle aspiration (TBNA). Peripheral lung lesions are easily detected with radial EBUS mini-probes. New generation of ultrasound processors with Power/Color Doppler and elastography seem to be helpful in the prediction of metastatic lymph-nodes during EBUS-TBNA. In the last 10 years several studies reported high diagnostic accuracy, minimal invasiveness and wide accessibility to hilar and mediastinal nodes of EBUS-TBNA (2). For these reasons both American College of Chest Physicians (ACCP) and European Society of Thoracic Surgeons (ESTS) guidelines recommended ultrasound guided needle aspiration as the best first diagnostic tool to obtain tissue for the staging of lung cancer (3,4).
EBUS-TBNA has been shown also to be a valuable technique to diagnose isolated mediastinal metastases in patients with previous malignancies with accuracy of 97.7% (5). Role of trans-bronchial needle aspiration under ultrasound guidance in diagnosis of benign disease and haematological malignancies is controversial. In single centre studies results in diagnosis of lymphoma and sarcoidosis seem to be dependent on patient selection criteria, local cytopathology service and size of sample (6,7).
Herein we analyze ten years experience with EBUS-TBNA in our Thoracic Surgery Department at Fondazione IRCCS Ca’ Granda University Hospital in Milan.
Methods
In our institution the first EBUS bronchoscope was available since 2005. Patients referred to our department with hilar and mediastinal lymph-nodes suspicious for malignancy were proposed for EBUS-TBNA after multidisciplinary meetings. Lymph-nodes with short axis diameter greater than 10 mm on contrast-enhanced computer tomography (CT) and/or metabolically active on positron emission tomography (PET) scan were considered as target for EBUS sampling.
Patients with high clinical suspect of lymphoma or sarcoidosis were not proposed for EBUS and scheduled for surgical biopsy.
Written informed consent was obtained from all patients before the procedure. All procedures were performed in a day-hospital setting. Topical local anaesthetic on vocal cords and oropharynx (lidocaine) was administered. Patients were placed under conscious sedation with midazolam and remifentanil by anesthesiologist on supine position, in association with nasal oxygen supplementation.
All procedures were performed using a flexible bronchoscope with linear convex probe with a frequency of 7.5 MHz (BF-UC160F-OL8, Olympus; Tokyo, Japan) and a dedicated 22-gauge needle (NA-201SX-4022, Olympus; Tokyo, Japan). Examinations were conducted in the operative theatre by a thoracic surgeon with the assistance of a resident or a nurse.
The nodal stations were classified according to the lymph node map of the 7th edition of TNM classification for lung cancer (8); staging of lung cancer nodes were sampled in order N3, N2 and N1 according to ESTS guidelines.
Each lymph node was punctured at least twice according to the quality of the specimen expelled from the needle: a long serpentine cast should be seen falling in the specimen collection system (Figure 1). In our experience no more than four passes were performed per nodal station in order not to last the procedure and cause discomfort to the patient.
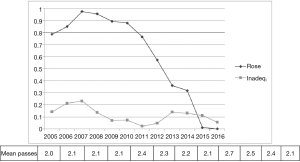
Rapid-on-site cytology evaluation (ROSE) was available only in part of the cases and in the last 5 years was used less frequently due to the skill achieved from different operators (Figure 2).
All the aspirated specimens were fixed in 10% formalin and paraffin embedded. Sections of 3 micron in thickness were cut and hematoxylin-eosin stained. In differentiated primary lung cancer the diagnosis was performed only at looking hematoxylin-eosin. In all remaining cases an immunohistochemical examination was made to verify glandular differentiation obtaining a P40 and P63 expression. When a suspicious of neuroendocrine differentiation appeared on hematoxylin-eosin slides, immunohistochemical markers as CD56, Chromogranin A or Synaptophisin were used to confirm the diagnostic hypothesis. In patients with history of previous malignancies the specimens were investigated with immunohistochemical panels oriented to primary tumor.
When molecular tests were requested by oncologist in order to guide the therapeutic management of the patients, analyses were performed on cell block derived material.
For mutational analysis of epidermal growth factor receptor (EGFR), Kirsten rat sarcoma (KRAS), v-Raf murine sarcoma viral oncogene homolog B (BRAF) and neuroblastoma RAS viral oncogene homolog (NRAS), a ratio of tumor cells/non-neoplastic cells ≥10% was considered acceptable. Anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) genes rearrangement tests and met proto-oncogene (MET) gene amplification test were performed in cases with at least 50 tumor cells.
EBUS-TBNA cytological results were assessed per patient. Inadequate specimen was defined in presence of an excess of red blood cells with no atypical or malignant cells identified, or if only bronchial cells were identified, or if the concentration of lymphocytes was not representative of lymph node tissue. The result of pathological examination was classified as negative when non-malignant cells (lymphocytes, histiocytes and anthracotic pigment) were detected in specimens.
Data are presented with numbers (percentages) for categorical variables and median [95% confidence interval (CI)] for continuous variables. The diagnostic sensitivity, specificity, positive predictive value (PPV), negative predicted value (NPV) and accuracy were calculated using standard definitions. A P-value <0.05 was considered significant. Statistical analyses were undertaken using MedCalc version 14.8 (Medcalc Software, Mariakerke, Belgium).
Results
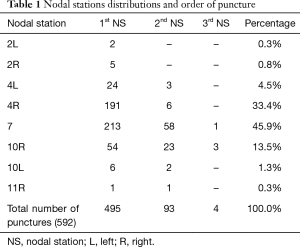
From October 2005 to August 2016, a total of 496 patients underwent EBUS-TBNA. Among them 348 (70.1%) were men, with median age of 67 (range 16–85) years. Number of hilar and mediastinal nodal stations punctured were 592 with a mean of 2.25 punctures per patient. In 93 patients a second nodal station was punctured and a third station in 4 cases. The most common site of transbronchial aspiration was the subcarinal station (272, 45.9%), followed by the lower right paratracheal station (197, 33.2%) (Table1).
Full table
No major complication has been recorded after the procedure. Bronchospasm occurred in two cases (0.4%).
Overall, inadequate procedures were 76 (15.3%). Twenty-eight patients with inadequate results were scheduled for a surgical biopsy and 48 patients were admitted to other hospitals and proposed for a different diagnostic procedure.
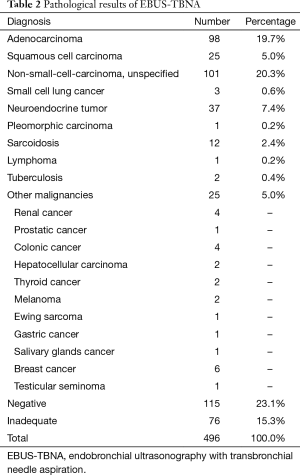
Diagnosis of malignancy was obtained in 291 patients (58.6%). The two most common pathologic histotypes were adenocarcinoma (98, 19.7%) and unspecified non-small cell lung cancer (NSCLC) (101, 20.3%). Diagnosis of nodal metastasis from an extrathoracic primary tumor was made in 25 cases (Table 2). In 14 patients (2.8%) the diagnosis was granulomatous diseases: 12 sarcoidosis and 2 tuberculosis. The presence of lymphocytes in specimens without malignant cells determined 115 negative cases (23.1%). These results were confirmed by surgical biopsies or clinical follow-up (CT or PET scan) in 83 patients. There were 13 false-negatives and 8 patients were lost during follow-up.
Full table
Sensitivity, specificity and diagnostic accuracy were 95%, 100% and 96% respectively. Negative predictive value was 90% and PPV was 100%.
ROSE was performed in 258 cases (52%) with median procedure duration of 30 minutes (95% CI, 30.0–37.7). In 238 patients (48%) ROSE was not available mainly for technical reasons and in this group median procedure duration was 30 minutes (95% CI, 30–35). In our series we recorded no difference in terms of length of EBUS procedure conducted with or without ROSE (Chi-squared test P=0.50). ROSE defined the procedure as adequate in 199 patients (77.1%). In this subset of patients the final diagnosis was accurate in 90.5% of the cases; a non-accurate final diagnosis occurred in 2%, and in 7.5% of the cases specimens were finally judged as inadequate. Fifty-eight patients had ROSE inadequate result which ended in a final accurate diagnosis in 69% of the cases, a non-accurate diagnosis in 3.4% while a definitive inadequate cytological finding occurred in 27.6% (P<0.001). Considering the whole population (496 patients) a final inadequate result was obtained in 12% of patients who received ROSE and in 13.9% of patients who did not (P=0.82).
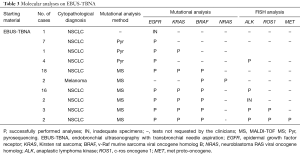
Molecular tests were requested in 54 NSCLC and 2 metastatic melanoma cases. Specifically, for the 52 NSCLC patients, the tests requested were: mutational status of EGFR (54 cases), KRAS (42 cases) and BRAF(39 cases), ALK rearrangement (27 cases), ROS1 rearrangement (5 cases) and MET amplification (2 cases). For the two melanoma cases, BRAF and NRAS mutational test was requested.
Out of 56 EBUS cases, only one specimen was not molecularly characterized, because the specimen was depleted for the cytopathologic diagnosis (Table 3). All the remaining 55 specimens had an appropriate tumor cells component for the mutational analysis (tumor cells/non-neoplastic cells ≥10%), and the DNA amount obtained from each tissue was sufficient for the amplification reactions.
Full table
Fluorescence in situ hybridization (FISH) test was requested for 27 EBUS cases, in all tissue specimens the tumor cellularity was appropriate for the analysis, as specified in the material and method section. In 25 out of 27 cases the analysis was completed, while in 2 cases the minimum tumor cells count for successful FISH testing was not achieved (Table 3), due to the consumption of the tissue specimens for the cytopathologic and the mutational analysis previously performed.
The mutational analysis performed on 53 NSCLC cases detected EGFR L858R mutation in 4 (7.5%) specimens. KRAS mutations were found in 5 out of 42 (11.9%) cases. Specifically, G12C and G13C mutations were seen in 2 cases, respectively, while G12V mutation was detected in 1 case. BRAF V600E mutation was detected in 1 out of 41 specimens.
One out of 27 cases was positive for ALK rearrangement (3.7%); MET amplification was seen in 1 out of 2 specimens, while all the 6 cases tested for ROS1 rearrangement were negative.
Regarding the two metastatic melanoma specimens, BRAF V600E mutation was detected in one case, while NRAS was not found mutated. The molecular results are reported in Table 4 and Table 5.

Full table

Full table
Overall, our data showed that the mutational analysis was successfully performed in 55 out of 56 EBUS specimens (98.2%), and FISH analysis in 26 out of 27 cases (96.2%). Only one specimen was completely depleted for cytopathologic diagnosis, not allowing the molecular characterization, and 3 out of 30 cases had no sufficient tumor cells number for appropriate FISH test.
Discussion
EBUS-TBNA in the past 10 years has become the method of choice in the staging of lung cancer and in the diagnosis of hilar and mediastinal lymphadenopathies. The main reason is that EBUS-TBNA is a safe procedure with high diagnostic yield that does not require general anesthesia and can be performed in an outpatient setting. In meta-analysis that includes 11 studies and 1,299 patients EBUS had a pooled diagnostic sensitivity of 93% and a pooled specificity of 100% (2). The diagnostic accuracy is lower in studies where patients are not selected on the basis of CT scan or PET scan positive results.
The availability of ROSE during EBUS-TBNA seems to reduce the rate of non-diagnostic sampling, the need for additional punctures and seems to increase sensitivity of the procedure (9,10). Even without rapid on-site cytological evaluation in several papers are reported diagnostic yield from 88% to 100% (11). A retrospective analysis on 131 patients underwent EBUS-TBNA with ROSE demonstrated that a final diagnosis was obtained in more than 73% of inadequate ROSE (12) and also in our series in 69% of inadequate ROSE an accurate diagnosis was obtained. On the contrary, even though the ROSE finding was adequate the final cytological result was inadequate in 7.5% of the cases. For those reasons we progressively reduced the use of ROSE during EBUS procedure without increasing the rate of inadequate cases (Figure 2).
We did not use EBUS-TBNA with the purpose of re-staging the mediastinum after induction therapy in lung cancer because diagnostic sensitivity is low (76%) and a NPV is unacceptable (20%) (13). After neoadjuvant chemotherapy to re-stage the nodal status we perform a surgical biopsy either by mediastinoscopy or by thoracoscopy.
Currently two different types of needle are available, 22-gauge and 21-gauge. A recent retrospective database analysis showed that there is no difference in specimen adequacy or diagnostic yield using needle with different diameters (14). When the procedure is performed with a 21-gauge needle adequacy with ROSE is achieved with less number of puncture. In our experience we prefer 22-gauge needle because the bronchoscope is more flexible and anatomical difficult nodal stations are easily reached.
The current standard of care for advanced lung cancer needs to identify key driver mutations and EBUS-TBNA has demonstrated to provide sufficient tissue for molecular testing (15). In our series the results are similar to those published in literature even if we do not have ROSE availability for all cases.
The EBUS-TBNA is a well tolerated procedure, complications are rare and similar to standard bronchoscopy. Major complications are reported with a rate lower than 0.1% (2). The bleeding complication is much less likely with EBUS-TBNA then blind TBNA as the procedure is performed with real-time visualization of the needle.
Conclusions
In our experience EBUS-TBNA is a safe, accurate and well tolerated procedure with high diagnostic yield to diagnose intrathoracic malignancies. In the era of targeted therapies for NSCLC, EBUS-TBNA remains the first choice tool for diagnosis, staging and treatment planning especially in lung cancer patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective observational cohort study has been approved by Ethics Committee of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico in Milan. Written informed consent was obtained from all patients before the procedure.
References
- Hürter T, Hanrath P. Endobronchial sonography: feasibility and preliminary results. Thorax 1992;47:565-7. [Crossref] [PubMed]
- Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer 2009;45:1389-96. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Nosotti M, Tosi D, Palleschi A, et al. Transbronchial needle aspiration under direct endobronchial ultrasound guidance of PET-positive isolated mediastinal adenopathy in patients with previous malignancy. Surg Endosc 2009;23:1356-9. [Crossref] [PubMed]
- Kennedy MP, Jimenez CA, Bruzzi JF, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lymphoma. Thorax 2008;63:360-5. [Crossref] [PubMed]
- Wong M, Yasufuku K, Nakajima T, et al. Endobronchial ultrasound: new insight for the diagnosis of sarcoidosis. Eur Respir J 2007;29:1182-6. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Nakajima T, Yasufuku K, Saegusa F, et al. Rapid on-site cytologic evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for nodal staging in patients with lung cancer. Ann Thorac Surg 2013;95:1695-9. [Crossref] [PubMed]
- Micames CG, McCrory DC, Pavey DA, et al. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: A systematic review and metaanalysis. Chest 2007;131:539-48. [Crossref] [PubMed]
- Anantham D, Koh MS, Ernst A. Endobronchial ultrasound. Respir Med 2009;103:1406-14. [Crossref] [PubMed]
- Joseph M, Jones T, Lutterbie Y, et al. Rapid on-site pathologic evaluation does not increase the efficacy of endobronchial ultrasonographic biopsy for mediastinal staging. Ann Thorac Surg 2013;96:403-10. [Crossref] [PubMed]
- Herth FJ, Annema JT, Eberhardt R, et al. Endobronchial ultrasound with transbronchial needle aspiration for restaging the mediastinum in lung cancer. J Clin Oncol 2008;26:3346-50. [Crossref] [PubMed]
- Yarmus LB, Akulian J, Lechtzin N, et al. Comparison of 21-gauge and 22-gauge aspiration needle in endobronchial ultrasound-guided transbronchial needle aspiration: results of the American College of Chest Physicians Quality Improvement Registry, Education, and Evaluation Registry. Chest 2013;143:1036-43. [Crossref] [PubMed]
- Jurado J, Saqi A, Maxfield R, et al. The efficacy of EBUS-guided transbronchial needle aspiration for molecular testing in lung adenocarcinoma. Ann Thorac Surg 2013;96:1196-202. [Crossref] [PubMed]