Uniportal video-assisted thoracoscopic surgery left upper lobectomy and systematic lymph node dissection with fused fissure
Introduction
The evolution of minimally invasive surgery is defined by overlapping epochs. Since the first multiport video-assisted thoracoscopic lobectomy was reported in 1992 (1) and the first uniportal video-assisted thoracoscopic lobectomy was demonstrated in 2011 (2), subsequent advances in techniques, technologies, and the proficiency of surgeons have accelerated the transformation of conventional open thoracotomy, three-port video-assisted thoracoscopic surgery (VATS), and double-port VATS to single-port VATS. A number of large institutional studies, multi-institutional registries, and meta-analyses have demonstrated the perioperative safety and long-term oncological efficacy of VATS lobectomy for patients with early stage non-small cell lung cancer (NSCLC) (3-6). The spectrum of uniportal VATS indications is now almost equal to that of conventional VATS (7). There were many studies about left upper lobectomy and systemic lymph node dissection in recent decade. However, their procedures were modularized without describing refined techniques or operative improvements (8-12).The pursuit of improvement in surgical techniques keeps moving. Without the assistance of tedious instruments, in this operation, we performed left upper lobectomy and systematic lymph node dissection with fused fissure via uniportal VATS and found it to be a smooth and skillful procedure.
Clinical summary
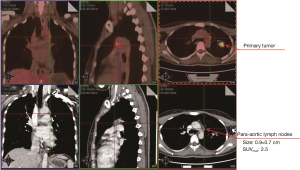
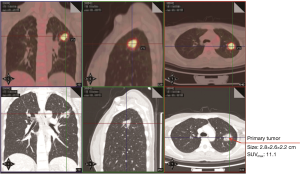
A 32-year-old female non-smoker was referred to Guangdong General Hospital, China following the detection of a solid nodule in the left upper lobe (LUL) during a routine medical examination. An 18F-Fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) scan revealed pleural retraction, and the size and maximum standardized uptake value (SUVmax) of the LUL lesion were 2.8 cm × 2.6 cm × 2.2 cm and 11.1, respectively (Figure 1). In addition, the PET/CT scan showed enlarged para-aortic a lymph node whose short axis was 0.7 cm with a low SUVmax of 2.5 (Figure 2). It didn’t meet the criteria of clinical N2 disease (short axis larger than 1 cm or SUVmax ≥2.5) (13). Based on the PET/CT scan, the LUL nodule was highly suspected to be lung cancer, and the clinical diagnosis was T2aN0M0, stage IB (8th edition of the TNM classification) (14). A suspicion of early stage primary malignancy led to LUL lobectomy and systematic lymph node dissection via uniportal VATS. The chest tube was removed on the 2nd postoperative day and the patient was discharged on the 3rd postoperative day with no complication.
Surgical techniques
Preoperative preparation
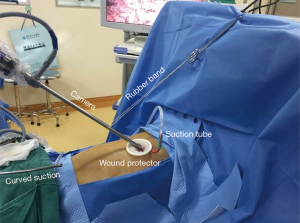
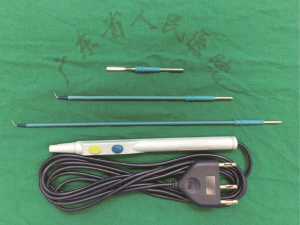
The operation was performed under general anesthesia with single-lung ventilation conducted by intubating a double-lumen endotracheal tube with the patient placed in the right lateral decubitus position. At the start of the operation, the surgeon stood on the ventral side of the patient and the sole assistant stood on the dorsal side; such a “face-to-face” position allowed the surgeon more room to maneuver. A single incision approximately 4 cm in length was created in the 5th intercostal space over the mid-axillary line, and a wound protector which could help reduce the risk of implantation metastases was routinely used without rib spreading. All of the procedures were performed with the assistance of a 10-mm 30-degree thoracoscopic video camera. The magnified visualization of the surgical field afforded by the thoracoscope is crucial for dissecting vessels or identifying small bleeders. The camera was fixed at the anterior edge of the incision by a rubber band to stabilize the thoracoscope and alleviate the assistant’s arm fatigue. The fixed camera also reduced interference when used in conjunction with other surgical instruments. A suction tube, formed from a manually modified small-sized catheter, was used to remove gas to maintain a clear view (Figure 3). The electrocautery hook and scalpel (Figure 4) designed by Xue-Ning Yang (15) was used during the sharp dissection of the thoracic anatomical structures, which differs from the blunt dissection created by a harmonic scalpel. When performing operations, surgeons use an electrocautery hook as if they were using a pen. Furthermore, surgeons can readjust the angle of the longitudinal axis according to individualized demands. Compared to the harmonic scalpel, our scalpel is more flexible, convenient, and fast-dissecting for uniportal VATS. Other instruments used frequently during the operation include a curved suction tube, articulated endoscopic staplers, vascular clips, and lymphatic node forceps. Considering the flexibility of manipulation, we became accustomed to using single joint dissectors or clamps.
Operative procedure
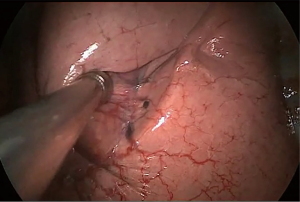
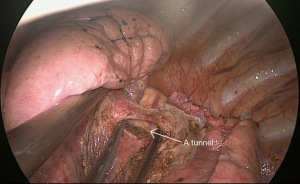
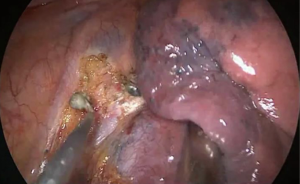
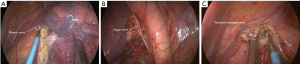
Surgery commenced by exploring the lesion located in the LUL and excluding unexpected intra-thoracic dissemination. We found a fused oblique fissure and palpably enlarged aortopulmonary (AP) zone lymph nodes and fused oblique fissure (Figure 5). The surgical strategy was decided based on the combined PET/CT scan and exploration, and a left upper lobectomy and systematic lymph node dissection were performed. At the beginning of the procedure, the posterior oblique fissure was detached using the electrocautery hook, cooperating with the curved suction tube. Next, the posterior mediastinal pleura was opened and a tunnel was created (Figure 6) to allow the fused oblique and mediastinal pleura to be perforated. When dividing a tissue bundle, the working tunnel gives priority to efficiency and safety. Nevertheless, it is crucial for surgeons to understand the precise anatomy and to identify no vessel and bronchus. The operation proceeded progressively from superficial to deep structures to expose the A1+2c (horizontalis) and A4 (lingualis superior) vessels. The tunnel was looped under the artery and encircled with thread and a clamp, and the stapler (ATW35; Ethicon Inc., Somerville, NJ, USA) was inserted through the tunnel to block the A1+2c and A4 arteries sequentially. Before cutting a vessel, it is worth confirming the distal end of the stapler, for safety. Then, the electrocautery hook was used to open the anterior mediastinal pleura and dissect the interlobar and hilar lymph nodes (stations 11 and 10) without clamping (Figure 7). Surgeons should be aware of the phrenic nerve (Figure 8A) to avoid damaging it. Careful non-clamping and en bloc lymphadenectomy was applied of the lymph nodes to avoid possible iatrogenic implantation. Then the left superior pulmonary vein was dissected and stapled (Echelon; Ethicon Inc., Somerville, NJ, USA). The site of incision, 5th costal space over the midaxillary line, contributed to lengthen the distance between surgical incision and superior pulmonary vein. The superior pulmonary vein was stapled at off-vertical angle with curved stapler. This facilitated the process of creating a tunnel from the anterior mediastinal pleura to the surface of the trunk of the left pulmonary artery to allow stapling of the incomplete anterior oblique fissure. We dissected and divided the A1+2a+b (apicalis + dorsalis) and A3 (ventralis) arteries. Before dividing the left upper bronchus, we inflated the left lower lobe to ensure that there was no stricture in the left lower bronchus; this was deemed an indispensable step in the operative procedure. Ventilator-related lung injuries should be avoided by ventilating with appropriate airway pressure. Thereafter, the LUL was removed using a specimen bag to minimize contact with the incision and eliminate the possibility of implantation metastases. The N1 (stations 11–13) lymph nodes of the resected lobe were anatomized and sent for pathologic examination. After sufficient tumor tissues had been obtained for pathological diagnosis, additional tumor tissues, paraneoplastic and normal lung tissues were resected for storage in our tumor specimen bank for subsequent analysis of gene profiles.
Returning to the dissection of the subcarinal lymph nodes to the contralateral main bronchus, preservation of the vagus nerve (Figure 8B) is crucial for ensuring physical function. The mediastinal pleura of the AP window (station 5) area was opened in a triangular shape, after which the AP window and para-aortic (station 6) lymph nodes were dissected. To protect the laryngeal nerve (Figure 8C), we found that reducing the power of the electrocautery hook to less than 30 watts were effective to avoid cutting or coagulation the nerve. The sequence of lymphadenectomy, from the unsuspicious lymph nodes to the suspicious, is crucial in reducing the possibility of pathological diagnosis interference and iatrogenic micro metastases. After systematic lymph node dissection based on standard principles, a 37 °C normal saline solution was used for chest washing, and a chest tube was positioned at the posterior edge of the single incision. Intradermal sutures were an effective measure to reduce cosmetic defects resulting from uniportal VATS.
Comments
Uniportal VATS has been adopted by many thoracic surgery departments in recent decades. Its safety and efficacy have been verified by a very sizeable volume of case reports, retrospective studies, a small number of clinical trials, and reviews (6,7,10,16-20). For technically challenging left upper lobectomy and systematic lymph node dissection, there is a desire to refine the surgical techniques constantly, create new operative tricks, and maximize the postoperative benefit. Our case was highly suspected to be primary lung cancer with low risky single-station metastasis. Hence, surgery was facilitated by the clinical diagnosis of stage IB disease and thoracic exploration. Firstly, a single incision was made at the mid-axillary line along the 5th intercostal space to allow a good access to the hilar structures and multiple lymph node stations. Due to the limitation of the single incision, the simultaneous use of a large number of instruments should be avoided. The combined use of the most frequently used instruments (a curved suction tube and an electrocautery hook) is of the utmost importance. The electrocautery, which combines the functions of cutting and coagulation, is an articulate instrument whose distal end can be converted into a hook or scalpel of different sizes, as required. Furthermore, the body of the electrocautery hook is ductile to allow for variable angulation and flexible dissection, thus overcoming the limitations of the harmonic scalpel. Second, a suction tube modified from a small-sized catheter was used for gas removal to maintain a clear view without narrowing the space. Encountering a misty camera lens and obscured view is a common occurrence for surgeons, and they obstruct the surgical procedure as well as endanger patients. Thus, a suction tube is a crucial auxiliary instrument in VATS. During our procedure, the thoracoscope was flexibly fixed by a rubber band. The tension of the rubber band and friction between the rubber band and camera serve to fix the camera in place; therefore, they not only reduce unwanted movement of the thoracoscope but also help to alleviate the assistant’s arm fatigue. A small-sized catheter and a rubber band, which are accessible and cost-effective, warrant wider application to VATS. Third, non-clamping lymphadenectomy, which could reduce traction and extrusion for lymph nodes, is a derivative of the tumor-free concept. Fractured metastatic lymph nodes might increase the risk of implantation and incomplete resection. Meanwhile, the sequence of lymphadenectomy is best executed from unsuspicious areas to suspicious areas. Although there was no evidence to confirm this ratiocination, we still encourage en block dissection of lymph nodes and operating from unsuspicious to suspicious metastatic areas. With experience gained from hundreds of uniportal video-assisted radical lobectomies, we summarize a strategy that it is effective and safe for creating a working tunnel before stapling structures such as vessels, bronchi, and fused fissures. Skeletonizing the target structures as far as possible can help reduce the quantity of endo-staples used. In addition, to some extent, it can also ease the financial burden for patients, particularly for economically challenging patients. Furthermore, eliminating obstruction makes the stapling procedure safer and more successful. Each improvement that is beneficial to patients deserves attention. Finally, preservation of the nerves should be observed by surgeons during the whole procedure to improve postoperative quality of life. The status of the lymph nodes is a paramount parameter in postoperative adjuvant therapy. To identify a complete and precise N1 stage, we recommend that surgeons subtly anatomize the N1 (stations 11–13) lymph nodes of a specimen.
Acknowledgements
Funding: This work was supported by grants from the National Natural Science Foundation of China (81001031, 81372285 and 81673031); the grant S2013010016354 from the Natural Science Foundation of Guangdong; The National Key Research and Development Program of China (Grant No. 2016 YFC 1303800); Guangdong Provincial Key Laboratory of Lung cancer Translation Medicine (Grant No. 2012A061400006); Special Fund for Research in the Public Interest from National Health and Family Planning Commission of PRC (Grant No. 201402031).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244-7. [PubMed]
- Gonzalez-Rivas D, de la Torre M, Fernandez R, et al. Single-port video-assisted thoracoscopic left upper lobectomy. Interact Cardiovasc Thorac Surg 2011;13:539-41. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Cao C, Manganas C, Ang SC, et al. A meta-analysis of unmatched and matched patients comparing video-assisted thoracoscopic lobectomy and conventional open lobectomy. Ann Cardiothorac Surg 2012;1:16-23. [PubMed]
- Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg 2010;139:976-81; discussion 81-3. [Crossref] [PubMed]
- Hsu PK, Lin WC, Chang YC, et al. Multiinstitutional analysis of single-port video-assisted thoracoscopic anatomical resection for primary lung cancer. Ann Thorac Surg 2015;99:1739-44. [Crossref] [PubMed]
- Akter F, Routledge T, Toufektzian L, et al. In minor and major thoracic procedures is uniport superior to multiport video-assisted thoracoscopic surgery? Interact Cardiovasc Thorac Surg 2015;20:550-5. [Crossref] [PubMed]
- Sawada S, Komori E, Yamashita M. Evaluation of video-assisted thoracoscopic surgery lobectomy requiring emergency conversion to thoracotomy. Eur J Cardiothorac Surg 2009;36:487-90. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fieira E, et al. Single-incision video-assisted thoracoscopic lobectomy: initial results. J Thorac Cardiovasc Surg 2012;143:745-7. [Crossref] [PubMed]
- Wang BY, Tu CC, Liu CY, et al. Single-incision thoracoscopic lobectomy and segmentectomy with radical lymph node dissection. Ann Thorac Surg 2013;96:977-82. [Crossref] [PubMed]
- Wang Q, Cai YX, Deng Y, et al. Modular 3-cm uniportal video-assisted thoracoscopic left upper lobectomy with systemic lymphadenectomy. J Thorac Dis 2016;8:2264-8. [Crossref] [PubMed]
- Feng M, Lin M, Shen Y, et al. Uniportal video-assisted thoracic surgery for left upper lobe: single-direction lobectomy with systematic lymphadenectomy. J Thorac Dis 2016;8:2281-3. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Zhai HR, Nie Q, Dong S, et al. Right upper lobectomy performed as dividing posterior ascending artery-bronchus-pulmonary vessels is alternative to primary indolent scar carcinomas. J Thorac Dis 2016;8:1340-4. [Crossref] [PubMed]
- McElnay PJ, Molyneux M, Krishnadas R, et al. Pain and recovery are comparable after either uniportal or multiport video-assisted thoracoscopic lobectomy: an observation study. Eur J Cardiothorac Surg 2015;47:912-5. [Crossref] [PubMed]
- Chung JH, Choi YS, Cho JH, et al. Uniportal video-assisted thoracoscopic lobectomy: an alternative to conventional thoracoscopic lobectomy in lung cancer surgery? Interact Cardiovasc Thorac Surg 2015;20:813-9. [Crossref] [PubMed]
- Harris CG, James RS, Tian DH, et al. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann Cardiothorac Surg 2016;5:76-84. [Crossref] [PubMed]
- Zhu Y, Liang M, Wu W, et al. Preliminary results of single-port versus triple-port complete thoracoscopic lobectomy for non-small cell lung cancer. Ann Transl Med 2015;3:92. [PubMed]
- Shen Y, Wang H, Feng M, et al. Single- versus multiple-port thoracoscopic lobectomy for lung cancer: a propensity-matched studydagger. Eur J Cardiothorac Surg 2016;49 Suppl 1:i48-53. [PubMed]