Long-term statin therapy could be efficacious in reducing the lipoprotein (a) levels in patients with coronary artery disease modified by some traditional risk factors
Introduction
Lipoprotein (a) [Lp (a)] is a well-established risk factor for coronary artery disease (CAD) (1-4). However, up till now, treatment of patients with higher Lp (a) levels is challenging. Apheresis technique is potent and effective in reducing the Lp (a) levels, but is also expensive and cumbersome. The promising drugs, including mipomersen (5,6), lomitapide (7) and protein convertase subtilisin/kexin type 9 (PCSK9) inhibitor (8,9) and antisense oligonucleotide (ASO) (10-12), are being intensively investigated, but are far from be widely used in clinical practice. Therefore, higher Lp (a) levels have often bogged down both doctors and patients. Statins are potent and effective low density lipoprotein cholesterol (LDL-C) lowering drugs that are widely used in clinical practice to reduce the incidence of CAD, myocardial infarction, stroke, peripheral vascular disease, and improve the outcome of revascularization procedures (13). However, studies regarding statin therapeutic effects on reduction of Lp (a) levels are conflicting. Some studies showed confirmative therapeutic effects of statin on the Lp (a) reductions (14-16). A recent meta-analysis also found that atorvastatin modestly decreased the Lp (a) levels (17). However, other studies didn’t find the statins’ beneficial effects on the Lp (a) reductions (18-20). We think that it’s reasonable the statins exhibit beneficial effects in reducing the Lp (a) levels given that the statins can deplete apolipoprotein B100 (apo B), which is indispensable to assemble the Lp (a). Furthermore, heterogeneities regarding patient enrollment, therapy duration and accompanied risk factors, which are neglected in the previous studies, may be the reasons causing the conflicting results. Therefore, it’s necessary to investigate the therapeutic effects of stains on the Lp (a) reductions from different angles before we obtain access to more reliable Lp (a) lowering drugs. The current study aimed to investigate the therapeutic effects of short, medium and long-term statin use on the Lp (a) reduction and its modifying factors.
Methods
The database
Case Collection and Scientific reSearch System for Clinical Cardiology (CCSSSCC) database has been described elsewhere (21). Briefly, this is a desk top database file system. The database now includes around 40,000 consecutive patients admitted in the Division of Cardiology ever since Jan. 1st, 2002. The establishment of and the access to this database has been approved by the Institutional Review Boards of the 1st Affiliated Hospital and the Soochow University (No. 2016SZYYLL00598). All patient medical records were anonymized and de-identified. The institutional Review Board waived the need for informed consent before analysis as the nature of these data was retrospective. The current study conforms to the principles outlined in the Declaration of Helsinki.
Patient selection
Consecutive CAD patients, aged ≥16 years old, homogeneous in Chinese Han ethnicity, abstracted from the database, admitted from Jan. 1, 2010 through Dec. 31, 2013, were included for potential analysis. Exclusion criteria: (I) those with thyroid abnormalities; (II) those with liver function abnormalities; (III) those with kidney function abnormalities or uremia; (III) those with coexistence of any entities mentioned above; (V) those with no Lp (a) examinations; and (VI) those with statin switch. Only first diagnosed CAD patients, without prior statin use at baseline, but with repeat hospitalizations in the follow-ups, were enrolled for final analysis in the current study so that we could obtain the lipid profiles at different times, and that we could rule out the effects of previously possible statin use. Patients were admitted because of chief complaint of chest discomfort, with a confirmatory diagnosis of CAD by CAG at baseline, and were readmitted during follow-ups because of recurrent chest discomfort or reexamination of stented or diseased vessels
Data on demographic factors, lifestyles, vital signs, comorbidities, blood glucose levels and lipid profiles were obtained. The data on height were missing in 3.44% of study subjects, fee in 0.34%, diastolic blood pressure (dbp) in 0.17%, and heart rate in 0.17% (Table 1).
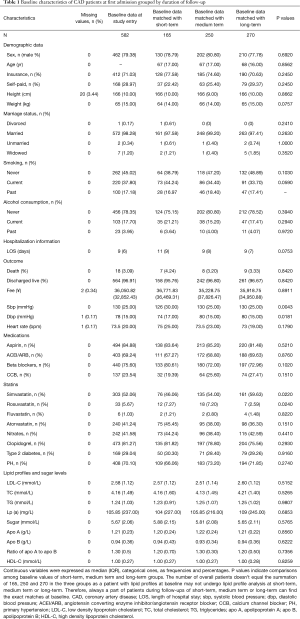
Full table
Definitions, diagnoses and grouping
Definitions of smoking, drinking, and body mass index, and diagnosis of CAD, primary hypertension (PH), type 2 diabetes mellitus (T2DM), thyroid and kidney dysfunction have been described elsewhere in details (21). Briefly, chief complaints, cardiac biomarker exams, echocardiography, treadmill exercise test, Holter monitoring, separately or in combination, were used for diagnosing CAD. Notably, the coronary angiography was 100% performed for these study subjects.
We took advantage of patients’ repeat hospitalizations to obtain the lipid profiles at different times. Short-term (median, 39 days) statin therapy was defined as greater than 7 days (inclusive) and less than 3 months (inclusive); medium term (median, 219 days), as greater than 3 months and less than 1 year (inclusive); and long-term (median, 677 days), as greater than 1 year. The statins and their dosages used in the current study are simvastatin with oral 40 mg once a day, atorvastatin with 20 mg, fluvastatin with 40 mg, and rosuvastatin with 10 mg. The lipid profiles before therapy served as baselines. At baseline, the first recordings were collected for a CAD patient with multiple lipid profile analyses. During follow-ups, the most recent recordings were collected for a patient with multiple lipid profile analyses in the same period of follow-up. Patients with Lipid profiles at short-term, medium term or long-term exactly matched with those at baseline. Every patient’s lipid profiles during the follow-ups were compared to his own ones at baselines. Thus, we made up the three before-after treatment groups.
Lp (a) measurement
The Lp (a) measurement method has been described in details elsewhere. Briefly, determination of the Lp (a) levels was performed using the latex-enhanced immunoturbidimetric diagnostic reagent kits. The assay range is 10–1,000 mg/L. The blood samples with Lp (a) levels >1,000 mg/L were routinely diluted 1:10. Thus, the Lp (a) concentrations up to 10,000 mg/L were within the security range of the assay and wouldn’t mistakenly be considered as a low level due to antigen excess. Lp (a) protein calibrator, in accordance with the IFCC PRM-2, provided by Sekisui Co. Ltd., has been used to calibrate the Lp (a) diagnostic reagents. The intra-assay and inter-assay coefficients of variation for Lp (a) were 2.5% and 3.11%, respectively.
Follow-ups
Repeat hospitalized patients with multiple lipid profile analyses were specifically focused as these patients made up an ideal population for a before-after study. CAD patient follow-ups after discharge were conducted in CAD clinics on a monthly basis for the first 4 months and on a bimestrial basis for the remaining 8 months, so as to know about patients’ symptoms, signs, drug administration and relevant imaging or lab examination results. For those who failed to visit the clinics, we called those patients or their relatives to know about the above-mentioned information. Particular attention has been paid to patients’ compliances with statin use. All information was written down in a standardized spreadsheet for future analysis.
Statistical analysis
Shapiro-Wilk test was hired for examining the characteristics of distribution of continuous variables. Continuous variables conforming to normal distribution were expressed as MEAN ± SD, otherwise, as median (interquartile range, IQR). Categorical variables were expressed as frequencies and percentages. Likelihood ratio chi squared test was used for comparison of frequencies or percentages among different groups. Kruskal-Wallis rank test was used for multiple independent sample comparisons as the variables were not distributed normally. Multilevel mixed effects model was used to compare changes of lipid profiles before and after-treatment. Patients were dichotomously divided according to presence or absence of some baseline characteristics, or arbitrarily divided into thirds in terms of LDL-C and HDL-C levels at baseline so as to further examine the effects of statin on Lp (a) levels in different stratifications.
Results
There were a total of 5,082 person-time CAD patients, among whom, 510 were excluded because of failure to examine Lp (a); 3,003, because of single Lp (a) exam; 101, because of thyroid dysfunction, kidney dysfunction and coexistence of any entities above; 19, because of single Lp (a) exam result left again due to exclusion of patients with above-mentioned morbidities; 27, because of failure to follow up or of undergoing any statin dosage adjustment during the follow-up; and 15, because of statin use duration <7 days. Thus, a total of 1,369 person-time CAD patients met for final analysis, from whom, we made up the three self-control groups: 165 pairs with short-term therapy; 250 pairs with medium term therapy; and 270 pairs with long-term therapy. Details were seen in flow diagram (Figure 1).
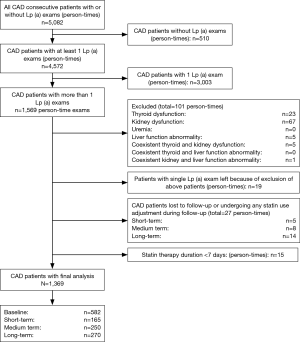
Baseline characteristics of CAD patients at initial admission grouped by duration of follow-up
The baseline characteristics of CAD patients were largely well-balanced among the three therapeutic groups. More patients received simvastatin treatment in medium or long-term group as compared with those (54%, 59% vs. 46%) in short-term one. In contrast, more patients received rosuvastatin treatment in short or medium term group as compared with those (7%, 7% vs. 2%) in long-term one. Use of fluvastatin or atorvastatin was similar (Ps>0.05) among different groups (Table 1).
Lipid profile [other than Lp (a)] changes after short, medium or long-term therapy vs. baseline values, grouped by statin use
The concentrations in LDL-C, TC, HDL-C and apo B were consistently and significantly decreased (Ps<0.05) after short, medium or long-term statin therapy as compared with baseline levels. The concentrations of ratio of apo A to apo B were consistently and significantly increased (Ps<0.05) after short, medium or long-term statin therapy as compared with baseline levels. The concentrations of TG were significantly decreased (Ps<0.05) after short- or medium term statin therapy as compared with those baseline levels. However, the therapeutic effects of statin on TG disappeared over a long-term statin use. The statin use had no any effects on apo A (Table 2).
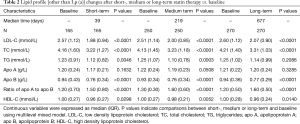
Full table
Lp (a) changes after short, medium or long-term therapy vs. baseline values, grouped by statin use
Overall, a trend towards lowering Lp (a) concentrations was consistently demonstrated after short-term, medium term or long-term statin therapy as compared with baseline levels. However, only after long-term statin therapy, did the trend reach the significant level (P=0.0011). When grouped by statin use, only long-term simvastatin therapy significantly decreased the Lp (a) levels as compared with baseline values. Atorvastatin, fluvastatin or rosuvastatin showed therapeutic effects on Lp (a), but without statistical significances (Table 3).

Full table
Lp (a) changes after short-, medium or long-term therapy vs0 baseline values, stratified by PH, DM, LDL-C and se.
Stratification analysis revealed that the Lp (a) levels were overall decreased in whatever follow-up durations in whatever stratifications. Interestingly, only in long-term statin use in some stratifications, were there significantly reduced Lp (a) levels as compared with baseline. The exception is in the mid tertile of LDL-C (2.18–3.03 mmol/L), where statin use increased the Lp (a) levels by ~10% across all durations of follow-up. Short-term and medium term statin therapy didn’t show any significant changes in Lp (a) levels in any stratifications (Table 4).

Full table
Discussions
The current study has demonstrated that long-term statin therapy significantly decreased the Lp (a) levels in CAD patients while short-term or medium term statin therapy didn’t. When grouped by statin use, only long-term simvastatin use significantly decreased the Lp (a) levels. PH, DM, LDL-C and HDL-C could modify the therapeutic effects of statin use on the Lp (a) levels in CAD patients.
As expected, the LDL-C, TC and apo B levels were markedly, consistently and significantly decreased after short-term, medium term or long-term statin therapy as compared with baselines, which were consistent with previous reports (22-24).
The findings in the current study that the short or medium term statin therapy didn’t reduce the Lp (a) levels have been lent support by several studies. Treatment with 20, 40, and 80 mg atorvastatin even for 24 weeks unexpectedly increased the Lp (a) levels by 9%, 8%, and 10% in CAD patients (19). MIRACL study also showed 16 weeks of treatment with 80 mg atorvastatin resulted in a significant increase of Lp (a) in acute coronary syndrome patients (20). A large, randomized, double blind and multicenter trial showed that simvastatin treatment for 24 weeks was not associated with a change in Lp (a) concentrations relative to placebo in primary hypercholesterolemia patients with LDL-C>190 mg/dL (18). In contrast, long-term statin therapy exhibited a significant reduction of Lp (a) level in several other studies, which was consistent with our findings. A 2-year, randomized, double blind trial revealed that treatment with 80 mg atorvastatin or 40 mg simvastatin significantly decreased the Lp (a) levels in familial hypercholesterolemia after 1 year and after 2 years (all P<0.0001), respectively. Atorvastatin therapy was more efficacious in reduction of Lp (a) levels (P=004) as compared with simvastatin at 1 year, but wasn’t any more (P=0.086) at 2 years (16). Another prospective, placebo-controlled randomized trial revealed that the Lp (a) levels were significantly decreased after 36 weeks atorvastatin therapy in dialysis patients (15). Our study also showed that only simvastatin exhibited beneficial effects of reduction of Lp (a) levels, which were different from prior studies (14-16). But we didn’t think simvastatin was different from atorvastatin in reduction of Lp (a) levels as the sample size in the current study was smaller in atorvastatin treatment group than in simvastatin one (98 vs. 161) with no sufficient statistical power to negate atorvastatin’s beneficial effects on Lp (a) reduction.
Statins inhibit cholesterol synthesis and activate LDL receptor, thus decreasing the levels in LDL-C and apo B, among which, apo B is a vehicle and a ligand for transporting and degrading endogenous cholesterol synthesized in liver (25,26). Apo B is also an indispensable component for assembling the Lp (a), which includes apolipoprotein (a) and LDL-C particle bound covalently by a disulfide between apolipoprotein (a) and apo B of LDL (27). The residence time of Lp (a) is longer than that of apo B in plasma (28). The inhibitory effects of statin on cholesterol synthesis are more pronounced than on apo B, or it takes more time to decrease the apo B levels, resulting in Lp (a) reductions, which may in part explain the findings revealed in the current study that only the long-term statin use is able to decrease the Lp (a) levels. We have to admit that the precise mechanism of the long-term statin use on Lp (a) reductions remains to be elucidated.
The current study revealed that risk factors, such as PH, DM, LDL-C and HDL-C, could modified the effects of statin use on Lp (a) reductions in patients with CAD. That higher Lp (a) levels in hypertensive patients were found than in normal controls seemed to support our findings in the stratification analysis that statin therapy could decrease the Lp (a) levels in hypertensive patients, but couldn’t in the normotensive patients (29). The higher Lp (a) levels would be more easily blunted by drug intervention. This explanation has been further supported by the findings revealed in the current study that only non DM patients’ Lp (a) levels were significantly decreased by statin treatment. Several studies showed that the Lp (a) levels were inversely associated with incident DM, which we think also partly explained the stratifying effects by DM in the current study (30-32).
Unexpectedly, in the mid tertile of LDL-C levels, the statin therapy almost consistently increased the Lp (a) levels across the short, medium and long-term groups, and reached the significant levels (P=0.0134) in the increase of the Lp (a) levels after long-term therapy. In contrast, the statin therapy insignificantly decreased the Lp (a) levels in the bottom or the top tertile of LDL-C levels across the short, medium and long-term groups. Similarly, in normotensive patients, the short or medium statin therapy was almost unchanged in the Lp (a) level reductions as compared with baselines. In contrast, the long-term statin therapy increased the Lp (a) levels although it didn’t reach the significant levels (118.4 vs. 128, P=0.1654). We cannot explain these phenomena, but they remind us it may be important to treat those high Lp (a) level patients with statins based on patients’ different risk factors (33).
Limitations
Our study has several limitations: first, observational study is susceptible to confounding factors, which may attenuate or exaggerate the observed effects of statin use on Lp (a) levels. Strictly matched pair wise analysis minimized these influences. Second, the current study selected the patients with multiple hospitalizations, and the majority of patients in the current study did not have repeat Lp (a) measured thus, the selection bias is potential and the generalization of our results should be cautious. Finally, we did not stratify the patients according to the severity of diseased coronary vessels although all the patients in the current study underwent coronary angiography exam. The severity of diseased vessels will affect patients’ hospitalizations and therapeutic effects. Further study is warranted to confirm our findings.
In conclusion, the long-term statin therapy is efficacious in reducing the Lp (a) levels in CAD patients, which has been modified by some traditional risk factors. Administration of statins with sufficient time as well as appropriate selection of CAD patients according to risk factors may be important so as to obtain the beneficiary effects of the Lp (a) reductions in CAD patients. We think the statin use duration > 1 year is appropriate based on the current study and previous reports (15, 16). In the era of commercial unavailability of more reliable Lp (a) lowering drugs, our findings will bolster confidence in fighting higher Lp (a) abnormalities for both patients and doctors. Large sample sized studies, specifically focusing on therapeutic effects of long-term statin use on the Lp (a) levels, were warranted to confirm our findings.
Acknowledgements
Funding: The current project has been in part supported by the National Natural Science Foundation of China (grant No. 81472918).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The establishment of and the access to this database has been approved by the Institutional Review Boards of the 1st Affiliated Hospital and the Soochow University (No. 2016SZYYLL00598). The institutional Review Board waived the need for informed consent before analysis as the nature of these data was retrospective.
References
- Luc G, Bard JM, Arveiler D, et al. Lipoprotein (a) as a predictor of coronary heart disease: the PRIME Study. Atherosclerosis 2002;163:377-84. [Crossref] [PubMed]
- Kamstrup PR, Benn M, Tybjaerg-Hansen A, et al. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation 2008;117:176-84. [Crossref] [PubMed]
- Erqou S, Kaptoge S, Perry PL, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 2009;302:412-23. [Crossref] [PubMed]
- Schaefer EJ, Lamon-Fava S, Jenner JL, et al. Lipoprotein(a) levels and risk of coronary heart disease in men. The lipid Research Clinics Coronary Primary Prevention Trial. JAMA 1994;271:999-1003. [Crossref] [PubMed]
- Akdim F, Visser ME, Tribble DL, et al. Effect of mipomersen, an apolipoprotein B synthesis inhibitor, on low-density lipoprotein cholesterol in patients with familial hypercholesterolemia. Am J Cardiol 2010;105:1413-9. [Crossref] [PubMed]
- Visser ME, Wagener G, Baker BF, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, lowers low-density lipoprotein cholesterol in high-risk statin-intolerant patients: a randomized, double-blind, placebo-controlled trial. Eur Heart J 2012;33:1142-9. [Crossref] [PubMed]
- Samaha FF, McKenney J, Bloedon LT, et al. Inhibition of microsomal triglyceride transfer protein alone or with ezetimibe in patients with moderate hypercholesterolemia. Nat Clin Pract Cardiovasc Med 2008;5:497-505. [Crossref] [PubMed]
- Stein EA, Mellis S, Yancopoulos GD, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med 2012;366:1108-18. [Crossref] [PubMed]
- Kotani K, Banach M. Lipoprotein(a) and inhibitors of proprotein convertase subtilisin/kexin type 9. J Thorac Dis 2017;9:E78-82. [Crossref] [PubMed]
- Visser ME, Witztum JL, Stroes ES, et al. Antisense oligonucleotides for the treatment of dyslipidaemia. Eur Heart J 2012;33:1451-8. [Crossref] [PubMed]
- Viney NJ, van Capelleveen JC, Geary RS, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet 2016;388:2239-53. [Crossref] [PubMed]
- Tsimikas S, Viney NJ, Hughes SG, et al. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet 2015;386:1472-83. [Crossref] [PubMed]
- Pasternak RC, Smith SJ, Bairey-Merz CN, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol 2002;40:567-72. [Crossref] [PubMed]
- Gonbert S, Malinsky S, Sposito AC, et al. Atorvastatin lowers lipoprotein(a) but not apolipoprotein(a) fragment levels in hypercholesterolemic subjects at high cardiovascular risk. Atherosclerosis 2002;164:305-11. [Crossref] [PubMed]
- Joy MS, Dornbrook-Lavender KA, Chin H, et al. Effects of atorvastatin on Lp(a) and lipoprotein profiles in hemodialysis patients. Ann Pharmacother 2008;42:9-15. [Crossref] [PubMed]
- van Wissen S, Smilde TJ, Trip MD, et al. Long term statin treatment reduces lipoprotein(a) concentrations in heterozygous familial hypercholesterolaemia. Heart 2003;89:893-6. [Crossref] [PubMed]
- Takagi H, Umemoto T. Atorvastatin decreases lipoprotein(a): a meta-analysis of randomized trials. Int J Cardiol 2012;154:183-6. [Crossref] [PubMed]
- Haffner S, Orchard T, Stein E, et al. Effect of simvastatin on Lp(a) concentrations. Clin Cardiol 1995;18:261-7. [Crossref] [PubMed]
- Schaefer EJ, McNamara JR, Tayler T, et al. Effects of atorvastatin on fasting and postprandial lipoprotein subclasses in coronary heart disease patients versus control subjects. Am J Cardiol 2002;90:689-96. [Crossref] [PubMed]
- Tsimikas S, Witztum JL, Miller ER, et al. High-dose atorvastatin reduces total plasma levels of oxidized phospholipids and immune complexes present on apolipoprotein B-100 in patients with acute coronary syndromes in the MIRACL trial. Circulation 2004;110:1406-12. [Crossref] [PubMed]
- Cai DP, He YM, Yang XJ, et al. Lipoprotein (a) is a risk factor for coronary artery disease in Chinese Han ethnic population modified by some traditional risk factors: A cross-sectional study of 3462 cases and 6125 controls. Clin Chim Acta 2015;451:278-86. [Crossref] [PubMed]
- Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670-81. [Crossref] [PubMed]
- Brugts JJ, Yetgin T, Hoeks SE, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ 2009;338:b2376. [Crossref] [PubMed]
- Mills EJ, Rachlis B, Wu P, et al. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Am Coll Cardiol 2008;52:1769-81. [Crossref] [PubMed]
- Scharnagl H, Schinker R, Gierens H, et al. Effect of atorvastatin, simvastatin, and lovastatin on the metabolism of cholesterol and triacylglycerides in HepG2 cells. Biochem Pharmacol 2001;62:1545-55. [Crossref] [PubMed]
- Boren J, Lee I, Zhu W, et al. Identification of the low density lipoprotein receptor-binding site in apolipoprotein B100 and the modulation of its binding activity by the carboxyl terminus in familial defective apo-B100. J Clin Invest 1998;101:1084-93. [Crossref] [PubMed]
- Kostner GM, Gavish D, Leopold B, et al. HMG CoA reductase inhibitors lower LDL cholesterol without reducing Lp(a) levels. Circulation 1989;80:1313-9. [Crossref] [PubMed]
- Krempler F, Kostner GM, Bolzano K, et al. Turnover of lipoprotein (a) in man. J Clin Invest 1980;65:1483-90. [Crossref] [PubMed]
- Serban C, Nicola T, Mateescu R, et al. Serum lipoprotein (a) levels in patients with arterial hypertension. Rev Med Chir Soc Med Nat Iasi 2010;114:798-802. [PubMed]
- Mora S, Kamstrup PR, Rifai N, et al. Lipoprotein(a) and risk of type 2 diabetes. Clin Chem 2010;56:1252-60. [Crossref] [PubMed]
- Rainwater DL, Haffner SM. Insulin and 2-hour glucose levels are inversely related to Lp(a) concentrations controlled for LPA genotype. Arterioscler Thromb Vasc Biol 1998;18:1335-41. [Crossref] [PubMed]
- Neele DM, de Wit EC, Princen HM. Insulin suppresses apolipoprotein(a) synthesis by primary cultures of cynomolgus monkey hepatocytes. Diabetologia 1999;42:41-4. [Crossref] [PubMed]
- Afshar M, Pilote L, Dufresne L, et al. Lipoprotein(a) Interactions With Low-Density Lipoprotein Cholesterol and Other Cardiovascular Risk Factors in Premature Acute Coronary Syndrome (ACS). J Am Heart Assoc 2016;5. [Crossref] [PubMed]


