Non-cardiac surgery following drug-eluting coronary stent implantation—a question of timing?
“Dear colleague, this 68 year old diabetic lady recently underwent percutaneous coronary intervention with drug eluting stents. Unfortunately, she now requires hip replacement surgery. I would be grateful for your advice regarding the optimal timing of surgery …”
This clinical scenario will be familiar to many involved in the management of patients undergoing non-cardiac surgery. Observational data indicate that between 5% and 35% of patients receiving a drug eluting stent (DES) undergo non-cardiac surgery within 1–2 years of their index percutaneous coronary intervention (PCI). Within this cohort, retrospective observational studies report an overall incidence of peri-operative death or major adverse cardiac events of 2–9%, with the greatest risk when surgery is performed early after stent implantation (1-4). Based on these data, the American College of Cardiology and American Heart Association focused update on duration of dual antiplatelet therapy in patients with coronary artery disease (5) recommends that non-cardiac surgery should be delayed for at least three (class IIb recommendation), but ideally for 6 months (class IIa recommendation), while the most recent European Society of Cardiology guidelines recommend delaying surgery for 6 months following DES implantation (6).
Besides their retrospective observational design, major limitations of previous studies have been the lack of a meaningful comparator or control population and the absence of detailed data on peri-operative antiplatelet use. Without these, it remains unclear to what degree the observed rate of peri-operative cardiac events is attributable to the presence of a DES, existing ischaemic heart disease, premature interruption of dual antiplatelet therapy, or surgical factors such as the urgency, type or magnitude of surgery undertaken. A recent study published in the Journal of the American College of Cardiology goes some way towards addressing these limitations and provides further insight into the relationship between the timing of surgery and peri-operative cardiac risk.
Egholm and colleagues undertook a retrospective data linkage study using the Western Denmark Heart and Danish National Patient Registries between 2005 and 2012 to identify 4,303 patients who underwent surgery within 12 months of DES implantation, and 20,032 patients without known ischaemic heart disease who underwent similar surgical procedures (7). Clinical outcomes at 30 days (myocardial infarction, cardiac death and all cause mortality) for each DES-PCI patient were compared with up to five control patients matched for age, sex and type of operation. Within the PCI cohort, use of first generation DES was high (60%) with just over half of the patients (56%) undergoing stent implantation for an acute coronary syndrome.
Consistent with previous studies (2,4), surgery performed within 1 month of DES implantation, emergency surgery, and acute coronary syndrome as the original indication for PCI were the strongest predictors of both acute myocardial infarction and death. Rates of myocardial infarction, cardiac and all cause death were 7.2%, 5.0% and 9% respectively for surgery performed within one month of DES implantation, compared with 0.5%, 0.4% and 2.1% respectively where surgery was performed between 1 and 12 months post PCI. Although no excess hazard was observed when surgery was performed beyond 1 month, the low frequency of events beyond this time point (myocardial infarction, n=18; cardiac death, n=13) may have limited the statistical power of the study.
The novel aspect of this study arises from the inclusion of a comparator population in an effort to address the issue of “confounding by indication”. Compared to patients without known ischaemic heart disease undergoing comparable operations, the DES-PCI cohort had higher rates of myocardial infarction (0.2% vs. 1.5%) and cardiac death (0.2% vs. 1.1%) and these differences were most marked where surgery was performed within one month of DES implantation [OR 14.3 (7.5–27.4) for MI; OR 15.1 (6.9–33.3) for cardiac death]. Importantly, in the control cohort matched with DES-PCI patients undergoing early surgery there was an increase in all cause mortality, but not myocardial infarction or cardiac death. Thus, some of the early excess hazard likely reflects the type and urgency of surgery undertaken and not just the presence of a DES.
Based on their findings, the authors conclude that surgery following DES-PCI may be safely considered sooner than is recommended in current guidelines (5,6). Before embracing this change, it should be highlighted that the majority of surgical procedures undertaken in the study were classified as low risk (>70%) and the number of clinical events observed beyond one month was low, limiting the statistical power of the study. Indeed, where surgery was performed between 1 and 2 months following DES-PCI, rates of MI (1.4% vs. 0.5%) and cardiac death (0.4% vs. 0.2%) were 2–3 fold greater in the DES cohort compared to control but these did not achieve statistical significance. Arguably, therefore, the findings of Egholm and colleagues are consistent with previous large scale studies demonstrating an excess in cardiac risk where surgery is performed early after PCI, with a reduction in risk over time, reaching a plateau at 3–6 months post DES-PCI (1,2,4,8) (Figure 1).
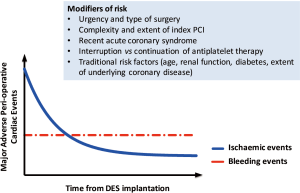
One important issue, closely linked to any decision regarding the timing of surgery following PCI but unanswered by the current study, is the optimal management of antiplatelet therapy in the peri-operative period. Any decision to interrupt dual antiplatelet therapy should be based on a careful evaluation of the risk of stent thrombosis balanced against the risk and consequences of peri-operative bleeding. In a registry of 666 patients with coronary stents undergoing non-cardiac surgery, discontinuation of antiplatelet therapy significantly increased the risk of peri-operative myocardial infarction and cardiac death without reducing the risk of major bleeding, although the majority of patients underwent surgery more than 6 months after PCI (9). Important factors to consider include: the lower risk of stent thrombosis with newer generation DES (10,11); randomised trials and pooled patient-level studies reporting that shorter durations (3–6 months) of dual antiplatelet therapy post DES-PCI are safe (12,13); registry data suggesting that physician guided, premature interruption of dual antiplatelet therapy may be performed for up to 14 days in patients with DES with no apparent increase in thrombotic risk (14).
Most agree that relatively minor surgical procedures can be undertaken safely without discontinuing antiplatelet therapy. Where premature interruption of dual antiplatelet therapy is necessary to allow surgery to proceed, consideration should be given to continuing aspirin alone. In a randomised trial of low dose aspirin in 10,010 patients undergoing non-cardiac surgery, POISE-2, peri-operative aspirin use was associated with a modest increase in major bleeding from 3.8% to 4.6% [HR 1.23; 95% CI, 1.01–1.49; P=0.04] with no effect on mortality (15). Robust data on the bleeding risk associated with dual antiplatelet therapy during major non-cardiac surgery are lacking. In one randomised trial of 108 patients undergoing major vascular surgery, there was no increase in major or minor bleeding but a greater need for blood transfusion, with aspirin and clopidogrel compared to aspirin alone (16). Bridging with low molecular weight heparin appears to be associated with increased bleeding and worse ischaemic outcomes and is not recommended (17).
Determining the optimum timing and management of antiplatelet therapy in patients requiring non-cardiac surgery following DES implantation is complex and does not lend itself easily to the conformity demanded by guidelines. Decision making frequently requires discussion between surgeons, cardiologists and anaesthetists alike with a careful evaluation of patient (age, renal function, vascular disease, recent acute coronary syndrome), procedural (interval from percutaneous coronary intervention, extent and complexity of DES-PCI, burden of disease), and surgical factors (urgency of indication for surgery, type of surgery, neuraxial blockade, need for interruption of antiplatelet therapy) (Figure 1). While the study by Egholm and colleagues (7) is unlikely to change current guidelines, it does offer physicians some reassurance that in selected, low risk patients where surgery cannot be delayed, surgery might be performed as early as 1 month following DES implantation with minimal excess hazard. On the question of antiplatelet therapy, only data from prospective randomized trials can inform these decisions; until this is available, clinicians must make individualized risk assessments on a case by case basis.
Acknowledgements
NLMC and MG are supported by NHS Research Scotland Career Researcher Awards.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hawn MT, Graham LA, Richman JS, et al. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013;310:1462-72. [Crossref] [PubMed]
- Tokushige A, Shiomi H, Morimoto T, et al. Incidence and Outcome of Surgical Procedures After Coronary Bare-Metal and Drug-Eluting Stent Implantation. Circ Cardiovasc Interv 2012;5:237-46. [Crossref] [PubMed]
- Gandhi NK, Abdel Karim AR, Banerjee S, et al. Frequency and risk of noncardiac surgery after drug-eluting stent implantation. Catheter Cardiovasc Interv 2011;77:972-6. [Crossref] [PubMed]
- Cruden NL, Harding SA, Flapan AD, et al. Previous coronary stent implantation and cardiac events in patients undergoing noncardiac surgery. Circ Cardiovasc Interv 2010;3:236-42. [Crossref] [PubMed]
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease. J Am Coll Cardiol 2016;68:1082-115. [Crossref] [PubMed]
- Kristensen SD, Knuuti J, Saraste A, et al. ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management. Eur Heart J 2014;35:2383-431. [Crossref] [PubMed]
- Holcomb CN, Graham LA, Richman JS, et al. The Incremental Risk of Noncardiac Surgery on Adverse Cardiac Events Following Coronary Stenting. J Am Coll Cardiol 2014;64:2730-9. [Crossref] [PubMed]
- Holcomb CN, Hollis RH, Graham LA, et al. Association of coronary stent indication with postoperative outcomes following noncardiac surgery. JAMA Surg 2016;151:462-9. [Crossref] [PubMed]
- Rossini R, Musumeci G, Capodanno D, et al. Perioperative management of oral antiplatelet therapy and clinical outcomes in coronary stent patients undergoing surgery. Thromb Haemost 2015;113:272-82. [Crossref] [PubMed]
- Palmerini T, Biondi-Zoccai G, Della Riva DD, et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet 2012;379:1393-402. [Crossref] [PubMed]
- Baber U, Mehran R, Sharma SK, et al. Impact of the everolimus-eluting stent on stent thrombosis: a meta-analysis of 13 randomized trials. J Am Coll Cardiol 2011;58:1569-77. [Crossref] [PubMed]
- Feres F, Costa RA, Abizaid A, et al. Three vs Twelve Months of Dual Antiplatelet Therapy After Zotarolimus-Eluting Stents. JAMA 2013;310:2510-22. [PubMed]
- Palmerini T, Sangiorgi D, Valgimigli M, et al. Short- versus long-term dual antiplatelet therapy after drug-eluting stent implantation: an individual patient data pairwise and network meta-analysis. J Am Coll Cardiol 2015;65:1092-102. [Crossref] [PubMed]
- Mehran R, Baber U, Steg PG, et al. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet 2013;382:1714-22. [Crossref] [PubMed]
- Eikelboom JW, Kearon C, Guyatt G, et al. Perioperative Aspirin for Prevention of Venous Thromboembolism. Anesthesiology 2016;125:1121-9. [Crossref] [PubMed]
- Burdess A, Nimmo AF, Garden OJ, et al. Randomized controlled trial of dual antiplatelet therapy in patients undergoing surgery for critical limb ischemia. Ann Surg 2010;252:37-42. [Crossref] [PubMed]
- Capodanno D, Musumeci G, Lettieri C, et al. Impact of bridging with perioperative low-molecular-weight heparin on cardiac and bleeding outcomes of stented patients undergoing non-cardiac surgery. Thromb Haemost 2015;114:423-31. [Crossref] [PubMed]


