Current costs of video-assisted thoracic surgery (VATS) lobectomy
Background
Video-assisted thoracic surgery (VATS) has been used more and more in daily practice for diagnosis and treatment of lung diseases especially non-small cell lung carcinoma in the last decade (1,2). Despite the growing enthusiasm for VATS resections, this minimally invasive technique has had slow adoption due to concerns regarding oncologic principles, costs, possible complications, time spent on learning curve and lack of surgeon training (3). Potential benefits of VATS for lung resections are listed in the literature as smaller incisions, less pain, less blood loss, less respiratory compromise, shortened hospital lengths of stay and at least similar survival rates (3,4). VATS lobectomy is oncologically the same surgical procedure as a lobectomy through a thoracotomy; both use anatomic resection, individual hilar ligation, and lymph node sampling or dissection (4). Several reports indicate that the number of dissected lymph nodes is similar between VATS lobectomy and thoracotomy (5,6), although other reports question this assertion. Five year survival rates are comparable and in at least several meta-analyses better (7,8). The greatest advantage of a VATS lobectomy may be an improvement in perioperative quality of life (4). According to Demmy and colleagues’ data, more patients who underwent thoracotomy required skilled nursing facilities after surgery (9) compared with a VATS approach. Several series have demonstrated that early postoperative pain is significantly less with VATS lobectomy (4,10). Patient who undergo VATS have a quicker recovery and have more strength to tolerate chemotherapy. As a result, theoretically, survival benefit will be higher if chemotherapy is started immediately after surgery (4). Postoperative pulmonary function also apears to be better after VATS than after a thoracotomy. In a nonrandomized comparison of patients who had a lobectomy by a thoracotomy or VATS, postoperative PaO2, O2 saturation, peak flow rates, forced expiratory volume in 1 second and forced vital capacity on both postoperative days 7 and 14 were better for the patients who had undergone the VATS procedure (11). The VATS patients have less impairment of pulmonary function and a better 6-min walk test than thoracotomy patients (12).
Recent data supporting advantages of VATS lobectomy
Several single institution series and a recent Society of Thoracic Surgeons (STS) database have demonstrated that compared with open thoracotomy, video-assisted thoracoscopic lobectomy may be associated with fewer postooperative complications (13). In the study of Paul et al. 73.8% of patients who underwent video-assisted thoracoscopic lobectomy had no complications, where as 65.3% of patients underwent lobectomy via thoracotomy had no complications. Compared with open lobectomy, video-assisted thoracoscopic lobectomy was associated with a lower incidence of arrhythmias, reintubation, blood transfusion as well as a shorter hospital stay and chest tube duration (13). In addition to these early functional advantages, video-assisted thoacoscopic lobectomy has been shown to have comparable long-term outcomes (14,15). The peri-operative advantages as well as the short and long-term outcomes reported have assuaged the concerns of the safety and efficacy aspects of video-assisted resections for the thoracic oncology patient population. However the drawbacks to VATS include higher equipment costs, longer operative room times and steeper learning curves for surgeons and operating room personnel (3).
Economic comparison of VATS versus open lobectomy
In a recent study our group compared hospital costs and perioperative outcomes for video-assisted thoracoscopic surgery and open lobectomy procedures in the United States using the Premier Prospective Database (Premier Inc, Charlotte, NC) (3). The study included the time period from the third quarter of 2007 through 2008. A total of 3,961 patients (open n=2,907, VATS n=1,054) were included in this evaluation. Length of stay was 7.83 days versus 6.15 days for open versus VATS. Surgery duration was shorter for open procedures at 3.75 versus 4.09 hours for VATS (Table 1) (3). The risk of adverse events was significantly lower in the VATS group (P=0.019) (3). Although statistically not significant, pneumonia occurred more frequently in the open group (9.1%) versus VATS (8.1%). Arrhythmias, other cardiac events and bleeding were found to be significantly more prevalent in the open group than in the VATS group. The frequency of patients with prolonged lengths of stay (>14 days) was higher in the open group than in the VATS group. Hospital costs were higher for open versus VATS; $21,016 versus $20,316 (P=0.027). Given that there is both a reduction in adverse events and a 1.68 day reduction in length of stay with VATS, one might expect the difference in cost between open and VATS to be greater than $700. Therefore, we looked at surgeon experience to determine if this played a role in cost. We examined surgeon experience with VATS over the 6 months prior to each operation and found a significant association between surgeon experience and cost. Average costs ranged from $22,050 for low volume surgeons to $18,133 for high volume surgeons. For open lobectomies, cost differences by surgeon experience were not significant and both levels were estimated at $21,000. These data suggest that economic impact is magnified as the surgeon’s experience increases.
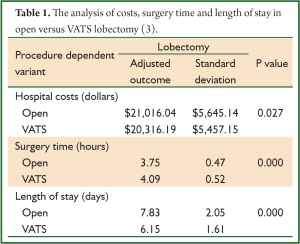
Full Table
In another recent retrospective study the relationship between volume and outcome in VATS surgery was evaluated (16). This relationship was striking for cost and utilization outcomes and VATS lobectomy as compared to VATS wedge resection. Outcomes following VATS surgery seems to be strongly associated with experience (16). This report showed that the reduction in cost and resource utilization increases significantly with greater experience and is most marked for VATS lobectomy for lung cancer. Moreover, thoracic surgeons have better VATS outcomes than non-thoracic surgeons and greater experience with open procedures does not correlate with better VATS outcomes. These findings reinforce the need for surgeons to focus on their VATS technique to achieve the best outcomes.
Another report on cost of VATS lobectomies revealed that the total hospital costs in the VATS group were lower than for those in the open lobectomy group ($5,391 vs. $5,593) (17). The reasons for the higher total hospital costs for open lobectomy were explained as longer hospital stays, longer chest tube duration and the need for more medications to control pain. Pulmonary complications, including respiratory dysfunction, pneumonia, atelectasis, empyema and prolonged air leak were less common with VATS approach in this series. A subset of patients in this group were compared according to the surgeon’s experience (early learning period vs. experienced learning period). Because of the decreased operation duration during the experienced learning period, the cost of anesthesia was significantly lower for these patients compared with those during the early period (17).
As the cost of surgical disposables play an important role in the total cost of VATS lobectomy, differences in the cost of resection of different lobes are also recorded (17,18). Casali and Walker demonstrated that upper lobectomy is more expensive than other types of lobectomy and that the difference in cost is mainly due to different need for the number of stapler cartridges (18). Cho demonstrated that the cost of surgical materials for resection of a lower lobe was lower than that for resection of the an upper lobe. The cost was $1,630 vs. $1,981 for right side and $1,655 vs. $1,908 for left side. When the total hospital costs were evaluated between the VATS lobectomy and open lobectomy groups for the five different lobes, VATS lobectomy for the left lower lobe was much more cost-effective than open lobectomy, although the difference was not statistically significant (17).
Using robotic technology to perform pulmonary surgery is of great current interest to the thoracic surgical community (19). Robotic lobectomies have been performed on a limited basis, with the advocates suggesting that the visualization and dissection are superior compared with a VATS approach. Robotic technology does have a certain appeal. The arms have a wrist-like movement and the magnification and depth of field of the robotic camera are superior to the standard VATS camera. However, it is not clear that these are significant advantages compared with VATS in the realm of cancer surgery. Compared with a VATS approach, the robotic incisions are the same size, the stapling instruments are the same, and the removal of the specimen is the same. The safety of VATS dissection of the vascular structures is excellent, with minimal reported problems after more than 17 years of experience. The completeness of lymph node dissection is complete with VATS and is not better with the robot, at least to date. Also, the surgical time and cost are significantly less for VATS (20). Robotic lobectomy has higher associated costs than VATS, primarily attributed to increased costs of the first hospital day, but it is less costly than thoracotomy approach for lobectomy (21). The average cost of VATS is substantially less than thoracotomy primarily because of a decreased length of stay. The cost of robotic assistance for VATS is still less than thoracotomy, but greater than VATS alone (21).
Conclusions
Minimally invasive techniques, such as VATS and robotics, are becoming the preferred approach in many surgical disciplines. Lobectomy performed by the VATS approach as compared with an open technique results in shorter length of stay, fewer adverse events and less overall cost. Patients who undergo VATS are discharged without home assistance and have low opiate requirements. Where there may be concern over the cost of the thoracoscopic equipment required for VATS, the significant hospital savings combined with better outcomes, particularly when an experienced surgeon performs the surgery, clearly favor the VATS approach over a thoracotomy. As the demand for health care resources increases, we must pay more attention to cost. Data, to date, shows a significant cost savings when a VATS approach is used compared to a thoracotomy for resection of lung cancer while enhancing short term outcomes and likely comparable or improved long term survival.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [PubMed]
- Swanson SJ, Herndon JE 2nd, D’Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [PubMed]
- Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg 2012;93:1027-32. [PubMed]
- Nicastri DG, Wisnivesky JP, Litle VR, et al. Thoracoscopic lobectomy: report on safety, discharge independence, pain, and chemotherapy tolerance. J Thorac Cardiovasc Surg 2008;135:642-7. [PubMed]
- Kondo T, Sagawa M, Tanita T, et al. Is complete systematic nodal dissection by thoracoscopic surgery possible? A prospective trial of video-assisted lobectomy for cancer of the right lung. J Thorac Cardiovasc Surg 1998;116:651-2. [PubMed]
- Sagawa M, Sato M, Sakurada A, et al. A prospective trial of systematic nodal dissection for lung cancer by video-assisted thoracic surgery: can it be perfect? Ann Thorac Surg 2002;73:900-4. [PubMed]
- Walker WS, Codispoti M, Soon SY, et al. Long-term outcomes following VATS lobectomy for non-small cell bronchogenic carcinoma. Eur J Cardiothorac Surg 2003;23:397-402. [PubMed]
- McKenna RJ Jr, Wolf RK, Brenner M, et al. Is lobectomy by video-assisted thoracic surgery an adequate cancer operation? Ann Thorac Surg 1998;66:1903-8. [PubMed]
- Demmy TL, Plante AJ, Nwogu CE, et al. Discharge independence with minimally invasive lobectomy. Am J Surg 2004;188:698-702. [PubMed]
- Nomori H, Horio H, Naruke T, et al. What is the advantage of a thoracoscopic lobectomy over a limited thoracotomy procedure for lung cancer surgery? Ann Thorac Surg 2001;72:879-84. [PubMed]
- Nakata M, Saeki H, Yokoyama N, et al. Pulmonary function after lobectomy: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2000;70:938-41. [PubMed]
- Nomori H, Ohtsuka T, Horio H, et al. Difference in the impairment of vital capacity and 6-minute walking after a lobectomy performed by thoracoscopic surgery, an anterior limited thoracotomy, an anteroaxillary thoracotomy, and a posterolateral thoracotomy. Surg Today 2003;33:7-12. [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [PubMed]
- D’Amico TA. Long-term outcomes of thoracoscopic lobectomy. Thorac Surg Clin 2008;18:259-62. [PubMed]
- Yamamoto K, Ohsumi A, Kojima F, et al. Long-term survival after video-assisted thoracic surgery lobectomy for primary lung cancer. Ann Thorac Surg 2010;89:353-9. [PubMed]
- David G, Gunnarsson CL, Moore M, et al. Surgeons’ volume-outcome relationship for lobectomies and wedge resections for cancer using video-assisted thoracoscopic techniques. Minim Invasive Surg 2012;2012:760292.
- Cho S, Do YW, Lee EB. Comparison of costs for video-assisted thoracic surgery lobectomy and open lobectomy for non-small cell lung cancer. Surg Endosc 2011;25:1054-61. [PubMed]
- Casali G, Walker WS. Video-assisted thoracic surgery lobectomy: can we afford it? Eur J Cardiothorac Surg 2009;35:423-8. [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [PubMed]
- Swanson SJ. Robotic pulmonary lobectomy--the future and probably should remain so. J Thorac Cardiovasc Surg 2010;140:954. [PubMed]
- Park BJ, Flores RM. Cost comparison of robotic, video-assisted thoracic surgery and thoracotomy approaches to pulmonary lobectomy. Thorac Surg Clin 2008;18:297-300, vii. [PubMed]


