Malignant tracheal necrosis and fistula formation following palliative chemoradiotherapy: a case report
Introduction
Radiotherapy and chemotherapy play important roles in both curative and palliative treatment of stages I to III non-small cell lung cancer (NSCLC) (1). During the course of lung cancer, the occurrence of a tracheomediastinal fistula cannot be easily predicted. Clinical data on tracheomediastinal fistula are limited to case reports (2-6). However, careful evaluation and proper management is needed because tracheomediastinal fistulas can be fatal.
Herein, we report a case of severe chondronecrosis of the distal trachea with formation of a fistula in a patient with metastatic lymphadenopathy stage IIIB NSCLC who received palliative concurrent chemoradiotherapy.
Case presentation
A 47-year-old man was referred to our hospital for evaluation of a right hilar mass identified in a chest X-ray scan. He presented with a 1-week history of dyspnea and coughing with sputum production and had lost approximately 15 kg of his body weight over the prior 6 months. He was a current smoker with a history of 23 pack-years and had a good performance status (ECOG 1). His vital signs were within normal limits, and his oxygen saturation was 95% on room air.
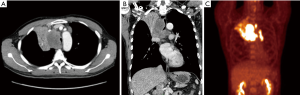
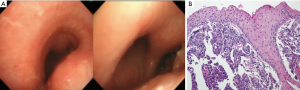
Chest computed tomography (CT) showed an 86.8 mm tumor invading the central airway (Figure 1). It appeared to be associated with multifocal conglomerated mediastinal lymphadenopathy, which caused narrowing of the distal trachea and right main bronchus. We performed an airway inspection using flexible bronchoscopy and observed an extrinsic tumor compressing the distal trachea of the right posterior side and right main bronchus (Figure 2A). The right bronchus was too narrow for passage of the bronchoscope; therefore, a tissue biopsy was performed of the right bronchus entry for pathological diagnosis. Microscopic examination led to a diagnosis of squamous cell carcinoma (Figure 2B). For staging workup, the patient was examined by positron emission tomography-CT and he was diagnosed with stage IIIB NSCLC (T4N3M0). A pulmonary function test revealed a forced vital capacity (FVC) of 3.8 L (75%), forced expiratory volume in 1 second (FEV1) of 1.08 L (26%), and a FEV1/FVC ratio of 28%.
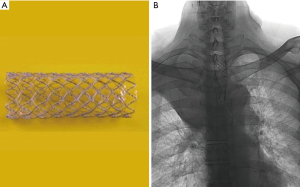
Palliative concurrent chemoradiotherapy was performed. The chemotherapy consisted of paclitaxel (45 mg/m2) and cisplatin (20 mg/m2) on days 1, 8, and 15 every 3 weeks. Once-daily thoracic radiotherapy was performed with a total dose of 35 Gy, 5 days a week, in 14 fractions. Prior to initiation of radiotherapy, an endotracheal metallic stent (HERCULES Airway stent, 18 mm/6 cm, Figure 3A) was inserted via an oral route at the stenosis site to prevent airway obstruction from stenosis aggravation, which can be caused by tumor edema following radiotherapy (Figure 3B). However, the patient experienced aggravated hypoxemia, and his oxygen saturation decreased to 68% with stridor. An emergency tracheal intubation was conducted in the ward and the patient was then transferred to the intensive care unit. A systemic steroid was prescribed to reduce the bronchial edema. The patient’s dyspnea was relieved, and airway intubation was successfully weaned the following day. After 1 week, the endotracheal stent was moved further away from the right insertion site but it caused a severe cough and therefore it was removed. After completion of the total 35 Gy (14 fractions) of radiotherapy, the patient was discharged and followed up as an outpatient.
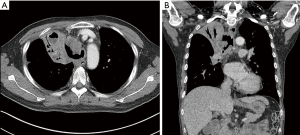
After 2 weeks, the patient was readmitted to the hospital complaining of aggravated dyspnea, cough, and sputum production. Chest CT showed a severe wall deformity with cartilage loss of the distal trachea and the presence of distal tracheal necrosis with a fistula extending into the right upper lung mass (Figure 4). Radiological response to treatment was a minimal response. Airway inspection with a flexible bronchoscopy revealed an improvement in the distal tracheal stenosis when compared with the initial bronchoscopic examination. However, the tracheal wall was completely disrupted on the right side of the distal trachea. The flexible bronchoscope passed through a cavity in the distal trachea that was filled with necrotic tissue exhibiting nodules covered with whitish exudates (Figure 5). Atypical squamous cells were discovered and the possibility of residual squamous cell carcinoma was indicated in the tissue biopsy specimen. Microbiological studies were performed on the tracheobronchial secretion, and Escherichia coli and Candida albicans were found in cultures.
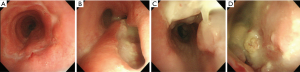
We were unable to perform surgical treatment or stent placement because the patient’s tumor was very large and his performance status was poor (ECOG 2). We provided the best supportive care available including antibiotics. However, the clinical course of postobstructive pneumonia tends to wax and wane. Eventually, he died 4 months after presenting with a tracheomediastinal fistula.
Discussion
Radiotherapy plays an essential role in both the curative and palliative treatment of stage I to III NSCLC. Curative radiotherapy is administered to patients with medically inoperable stage I to II NSCLC, and concurrent chemoradiotherapy is administered to patients with unresectable advanced stage III NSCLC. Palliative radiotherapy can relieve thoracic symptoms such as airway obstruction, chest pain, cough, and hemoptysis (1,7,8).
Tracheomediastinal fistulas after concurrent chemoradiotherapy for lung cancer are very rarely reported. Therefore, their incidence is unknown (2). Pulmonary resection, various infections, chemotherapy, radiotherapy, central located tumors, and squamous cell carcinoma may be risk factors (2,4).
The mechanism of tracheomediastinal fistula can be explained by the endarteritis and reactive fibrosis of the treated tissue, which compromises the tissue blood supply and produces a hypoxic environment within the targeted cancer cells and normal tissue surrounding the tumor. Tissue hypoxemia causes mucosal edema, inflammation, and perichondritis, and ultimately proceeds to cartilage necrosis (9). This process is accelerated by inflammation caused by hypoxemia, infection from respiratory tract bacteria, and injury to the tracheal wall. Other predisposing factors are tumor invasion, smoking, and a high dose of radiation (10). This mechanism might explain why the breakdown of the perichondrial membrane by radiotherapy and traumatic injury allows respiratory bacteria to access the cartilage membrane and how such infected tissues then progresses to necrosis, abscessation, and finally fistula formation. The necrosis of lymph nodes that initially compress the tracheal wall is thought to be the ultimate cause of fistula formation, as in our case (4).
Previous cases mostly reported late chondronecrosis and tracheoesophageal fistula after radiation therapy. Some of them reported that tracheomediastinal fistulas occur early after thyroidectomy in thyroid cancer or goiter (11). The risk factors are female sex, thyrotoxic goiter, wound infection, or excessive use of diathermy. The mechanism of fistula is that alteration of vascularity can cause ischemia around the trachea and accelerate necrosis combined with infection (11). A different and meaningful point in our case is that early severe tracheal necrosis occurred just after chemoradiation therapy without any apparent direct alteration of vascularity around the trachea, such as after a surgery. However, there were some combined risk factors leading to tracheal injuries in our case, including stent insertion, endotracheal intubation, compression by a large tumor mass, radiotherapy, and infection, and in combination they accelerated the chondronecrosis of the central trachea.
The diagnosis of a tracheomediastinal fistula is based on clinical presentation, chest CT, and bronchoscopy findings. The chest CT scan normally reveals tracheal and bronchial narrowing, anatomical distortion, and disruption of the airway cartilage. Bronchoscopy can detect early necrotic areas and mucosal edema that are barely able to be distinguished on the chest CT image as well as tracheal disruption. Therefore, fibrotic bronchoscopy can be performed to find early-stage necrotic tissue in suspected cases of lower respiratory tract fistula.
Radiotherapy for a medically inoperable central tumor in patients with NSCLC delivers a therapeutic median dose of 50 to 60 Gy, in consideration of efficacy and safety (7). Recent radiation technology has advanced to allow for accurate targeted radiation beams, which thus reduces the adverse effects on normal tissue; however, higher-dose radiotherapy can still cause complications (12). Although our patient was exposed to a palliative radiation dose of 35 Gy, which is less than the conventional dose, a tracheomediastinal fistula occurred. The fistula seems to be due to invasion by the large tracheal tumor and uncontrolled pneumonia, which developed simultaneously and facilitated progression of the necrosis of the tracheal wall.
The treatments for tracheomediastinal fistula include medical management, stent placement, and surgery. The treatment for chondronecrosis of the distal trachea can be decided depending on the location, extent, and depth of the chondronecrosis as well as the condition of the tumor (13). For example, tracheomediastinal fistula in the form of small ulcers can be treated with antibiotics, corticosteroids, argon plasma coagulation, and fibrin glue (4,14). Corticosteroids can reduce the severity of the inflammation and tracheal perichondritis. Some case reports used a self-expanding metallic stent (2,6,15,16). Complications caused by a stent insertion are related to the migration of the stent, granulation, and airway obstruction due to retention of secretions. Surgical end-to-end anastomosis is recommended for patients with severe necrosis, pneumomediastinum, or pneumothorax (17). However, this procedure had limited benefit in our case because of the high risk associated with the operation (13).
Tracheomediastinal fistulas can be a fatal and irreversible complication. Therefore, careful inspection of the airways in the early stage and proper medical management are important to prevent severe morbidities and mortalities. If advanced NSCLC takes the form of a central tumor adjacent to the trachea, concurrent chemoradiotherapy could be considered, but it may result in irreversible and fatal tracheomediastinal fistulas. Accordingly, the application of chemotherapy, radiotherapy alone, or sequential chemoradiotherapy should be considered to reduce toxicity prior to concurrent chemoradiotherapy. If concurrent chemoradiotherapy is performed, bronchoscopic evaluation could be considered to identify mucosal changes and enable the detection of early necrosis or tracheal disruption.
Although the radiation dose given to our patient was moderate, airway necrosis still occurred. In cases of centrally located lung cancers or malignant lymph nodes attached to the tracheal wall, careful evaluation with CT or bronchoscopy could help detect tracheal wall disruption after concurrent chemoradiotherapy. Careful evaluation, early detection, and timely intervention are important and are the best way to prevent and treat tracheomediastinal fistulas adequately.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent could not be obtained from the patient because the patient died before writing this manuscript. The Ethic Committee of our institution has waived the approval.
References
- Horn L, Pao W, Johnson DH. Neoplasms of the lung. In: Longo DL, Fauci AS, Kasper DL, et al. editors. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill, 2011:737-53.
- Machuzak MS, Santacruz JF, Jaber W, et al. Malignant tracheal-mediastinal-parenchymal-pleural fistula after chemoradiation plus bevacizumab: management with a Y-silicone stent inside a metallic covered stent. J Bronchology Interv Pulmonol 2015;22:85-9. [Crossref] [PubMed]
- Hakamoto J, Hasegawa S, Ito S, et al. A case of broncho-mediastinal fistula with primary lung cancer. J Bronchol 2004;11:42-4. [Crossref]
- Ucer M, Ordu C, Pilanc KN, et al. Tracheomediastinal fistula in a patient with lung adenocarcinoma and its treatment with argon plasma coagulation: a case report. Medicine (Baltimore) 2014;93:e156. [Crossref] [PubMed]
- McCarthy J, Hamel J. Tracheal-mediastinal fistula post-chemoradiation therapy. West J Emerg Med 2014;15:876-7. [Crossref] [PubMed]
- Ranes JL, Budev MM, Murthy S, et al. Management of tracheomediastinal fistulas using self-expanding metallic stents. J Thorac Cardiovasc Surg 2006;131:748-9. [Crossref] [PubMed]
- Senan S, Lagerwaard FJ. The role of radiotherapy in non-small cell lung cancer. Ann Oncol 2005;16 Suppl 2:ii223-8. [Crossref] [PubMed]
- Toy E, Macbeth F, Coles B, et al. Palliative thoracic radiotherapy for non-small cell lung cancer, systemic review. Am J Clin Oncol 2003;26:112-20. [Crossref] [PubMed]
- Cukurova I, Cetinkaya EA. Radionecrosis of the larynx: case report and review of the literature. Acta Otorhinolaryngol Ital 2010;30:205-8. [PubMed]
- Rowley H, Walsh M, McShane D, et al. Chondroradionecrosis of the larynx: still a diagnostic dilemma. J Laryngol Otol 1995;109:218-20. [Crossref] [PubMed]
- Conzo G, Fiorelli A, Palazzo A, et al. An unpredicted case of tracheal necrosis following thyroidectomy. Ann Ital Chir 2012;83:55-8. [PubMed]
- Corradetti MN, Haas AR, Rengan R. Central-airway necrosis after stereotactic body-radiation therapy. N Engl J Med 2012;366:2327-9. [Crossref] [PubMed]
- Aerni MR, Parambil JG, Allen MS, et al. Nontraumatic disruption of the fibrocartilaginous trachea: causes and clinical outcomes. Chest 2006;130:1143-9. [Crossref] [PubMed]
- Weiss G, Wex C, Lippert H, et al. Successful endoscopic treatment of a postoperative tracheomediastinal fistula caused by anastomotic insufficiency after esophageal resection with fibrin glue. Pol Przegl Chir 2015;86:537-9. [Crossref] [PubMed]
- Shin JH, Kim SB, Kim JH, et al. Management of tracheomediastinal fistula using a self-expanding metallic tracheal stent. Cardiovasc itevent Radiol 2009;32:843-5.
- Choudhary C, Gildea TR, Salman R, et al. Management of tracheomediastinal fistula using self-expanding metallic stents. Ann Thorac Surg 2008;85:1800-2. [Crossref] [PubMed]
- Alraiyes AH, Alraies MC, Abbas A. Radiation-associated airway necrosis. Ochsner J 2013;13:273-5. [PubMed]


