Pulmonary metastasectomy in uterine malignancy: outcomes and prognostic factors
Introduction
Metastatic uterine cancer is by definition a form of systemic disease and there is general agreement that it should be treated as such. While extensive spread to distant organs precludes any attempt of radicality, some authors advocate that in selected patients metastasectomy may indeed play a role in long-term disease control (1,2). As is the case with many other solid tumours, the practice of metastasectomy is open to criticism as there is lack of convincing evidence, and over-encouraging outcomes may be attributed to intrinsic selection bias (3). The accepted criteria for pulmonary metastasectomy include lesions that are completely resectable with acceptable operative risk, in the absence of extrapulmonary spread, when the primary tumour is completely controlled and when alternative or more effective treatment options are unavailable (1,2).
As to these conditions are infrequently all present in uterine cancer, the number of published reports (all retrospective and small) is scarce, and the likelihood of a randomised trial is effectively speculative. Comparative analysis of these studies is made even more difficult by a number of additional limitations. These include the variability of different tumour types with very different prognoses and a wide variety of treatments to control the primary tumour including any combination of surgery, chemotherapy and radiotherapy. Similarly, once pulmonary metastasis was apparent, a variety of complementary treatment options may be offered and with different timing, sometimes inconsistently within the same series, and with a different approach towards the extent of the lung resection and the use of repeated metastasectomy.
However, they all have merit in highlighting prognostic factors that may direct and inform an individualised patient decision.
Overview of epidemiology
In gynecologic cancers the rate of lung metastases is higher for choriocarcinoma and sarcoma than in patients with epithelioid cancer such as cervical, endometrial or ovarian, although these three are rather more frequent (4). All major studies report an average age of 55–59 years at the time of lung resection, with a wide range spanning from the very young age (<20) to the more elderly (>80) (5). About 80% of cases present with multiple or bilateral metastases, while synchronous metastases are reported in about 20% of cases (6).
Endometrial cancer is the most common tumour of the female genital tract in Western countries (4,7). Extra uterine spread is reported in about 25% of cases, most commonly through pelvic and paraaortic lymph nodes or pelvic organs such as peritoneum and adnexa. Lung dissemination is the most common site of extrapelvic disease, especially for locally advanced cases, although it may occur in early stage disease (4). It is however relatively rare with reports ranging between 2.3–4.6% of cases depending on the series (8).
On the other hand, cervical cancer is the most common gynecological cancer in developing countries, with a reported 1.8% of lung metastases in a study on 2,075 women in Sri Lanka (4). Isolated lung metastases are reportedly seen in 1.1–6% of cases (9). These figures drop to barely 0.37% in stage I–II cervical cancer, which is a far more common presentation than more advanced stages (10).
Uterine leiomyosarcoma accounts for only 1–3% of uterine cancers, however seems to contribute to about 25% off all uterine-cancer related deaths. Lung metastasis is much more common in as much as 30% of cases (11).
In other areas of gynecological oncology, ovarian cancer seems uniquely to exhibit a specific affinity to pleural spread (reflecting its mesothelial origin), presenting with effusion and pleural lesions rather than isolated pulmonary nodules (4). Likewise, choriocarcinoma (rarer) is usually chemo-sensitive, therefore lung resection is relatively unusual and reserved solely to chemoresistant oligometastatic disease (4).
History and outcomes of lung resection
The first case of a pulmonary resection for uterine adenocarcinoma was reported by Torek in 1930 (12).
A limited number of case reports or small series followed in the years (4,8,13), but the first larger surgical series to appear was a study from Memorial Sloan Kettering Cancer Centre in 1992 (14). Forty-five patients with pulmonary metastases from uterine sarcomas were resected (29% bilaterally). All had potentially resectable lesions but only 74% eventually had a complete resection. Five- and 10-year survival was respectively 65% and 50% from diagnosis and 43% and 35% from metastasectomy, with the only significant predictor of survival identified in unilateral versus bilateral location.
Since then, a number of surgical series as well as mixed series including both surgical and non-surgical patients have been published with sometimes controversial results.
In some mixed series including surgical and non-surgical patients, for example, oestrogen receptor status and the use of hormonal therapy seemed to be the only therapeutic option able to prolong survival, whereas neither surgery nor chemotherapy seemed to, with reported survival of 28, 18 and 14 months respectively (15,16). Likewise, in another Japanese study, patients who developed pulmonary recurrence 2 years after the initial therapy had a significantly longer survival than those who developed it within 2 years (31 versus 10 months, P=0.01), but this was regardless of whether they underwent lung resection or not (17). On the contrary, another series identified patients with 1–3 nodules, smaller than 3 cm and with negative local (abdominal) nodal metastases to be optimal candidates for lung resection (18).
Purely surgical series are limited in number
In 2001, a median survival of 26 months after lung resection for uterine body cancer was reported by Anderson, rising up to 46 months if the histology was adenocarcinoma. In the same series, a median survival of 36 months was seen if the tumour was instead originating from the cervix (13).
In 2004, 7,748 patients with stage IB or II cervical cancer who underwent curative initial treatment in 22 hospitals, were reviewed by Yamamoto and colleagues. Twenty-nine (0.37%) of these underwent subsequent resection for non-synchronous lung metastases. The results were quite polarised between patients who had less or more than 3 metastases (5-year survival of 42% vs. 0% respectively) and patients who had squamous versus non-squamous histology (5-year survival of 47% and 0% respectively) (10).
In the largest series published in 2004, 133 patients were reviewed from the Registry of the Metastatic Lung Tumour Study Group of Japan. In the whole cohort, 38 patients underwent bilateral metastasectomy (single-stage or two-stage), while other 12 patients underwent more than one and up to four repeated metastasectomies. Of note, this study included 8 patients (6%) with evidence of extrapulmonary metastatic disease. Overall 5- and 10-year survival was 54.6% and 44.9% respectively. Breaking down by histology, choriocarcinoma held the best 5-year survival (86%), followed by endometrial adenocarcinoma (76%), endometrial squamous cell carcinoma (47%), both adenocarcinoma and squamous cell carcinoma of the cervix (40%) and leiomyosarcoma (38%). Uterine body tumours seemed therefore to fare better than cervical cancers, largely dependent though on specific histology. Statistically significant negative prognostic factors were cervical location of primary tumour, and a disease-free interval (DFI) from primary tumour resection of less than 12 months (59.8% vs. 36.8%). The difference was even more striking after excluding a minor proportion (n=6) with synchronous metastases (59.8% vs. 17.1%). However, 5-year survival of patients with shorter DFI but only one metastasis was 48%, and in fact equally significant factors negatively affecting survival were number of metastases >4 and tumour size >3 cm.
About a third of the patients underwent mediastinal lymphadenectomy, 34% showing pathologically positive lymph nodes. Although they seemed to have a worse survival than those with negative nodes, no difference was found with those not undergoing lymphadenectomy (5).
In another large series published by the Mayo Clinic in 2006, 70 patients were reviewed with a median DFI of 24 months after primary gynaecological procedure; 7% had an incomplete lung resection. Overall 5- and 10-year survival was 46.8% and 34.3% respectively. However, median DFI after metastasectomy was only 8 months, with a 3-year disease-free survival of only 14.3%. Seventy-eight percent of patients eventually developed a recurrence, in about half of the cases involving again the lung. In almost all of these cases, a policy of repeated metastasectomy was adopted by this group. Although cervix represented only 10% of the total population, this was found again to be a negative predictive factor together with a DFI of less than 24 months. A total of 35 patients (50%) died, while 35 still were still alive after a median follow up of 36 months (19).
In a smaller Spanish series, 27 cases were analysed with a very long median disease free interval between diagnosis and metastasectomy of 58 months. Median survival after metastasectomy was 94 months with a 5-year survival of 84% (again largely better for endometrial than for cervical or sarcoma origin, 100% and 60% respectively) (7). Beyond the sensational results, these figures are however far from being effective supporting evidence in favour of metastasectomy, as they rather seem to point out quite eloquently in this case how the impact of selection bias can stretch the actual numbers.
In a more recent comparative study by Adachi et al., data for 37 patients with isolated lung metastases were retrospectively reviewed. A group of 23 patients who received lung resection and chemotherapy was identified and compared to a group of 14 patients who received non-surgical treatment only due to inoperable mass or chemoresistant nodules. This series only included epithelial tumours, including 5 of ovarian origin. Five-year survival, calculated from time of diagnosis, was 81.7% in the surgical group versus 49.5% in the non-surgical, although the difference was not statistically significant. Eight-year survival was again 81.7% versus 24% respectively. Similarly to previous studies, operated cervical cancer metastases seemed to do slightly worse (5-year survival 61%) compared to endometrial (100%). These survival figures are largely higher than what reported in other studies, which is probably in keeping with the restrictive exclusion criteria such as number of nodules >3 or synchronous metastases, already known to impact on survival.
Recurrence-free interval greater than 2 years was 100% in the surgical group versus 41.7% in the non-surgical. In the former, 5 of 6 lung re-recurrences who underwent further surgery were reported to be still alive at last follow up, although no specific intervals are provided (9).
In another recent series, 29 patients were reviewed. Despite the fact that histology was sarcoma in 41% of cases, that 41% had more than three nodules and 28% had metastases-related symptoms, 5-year for the whole cohort was still 48% with a median overall survival of 26 months. At multivariate and univariate analyses, presence of symptoms and number of metastases greater than 3 negatively affected survival, while DFI <12 months also did but not significantly (8).
The results of all these studies are briefly summarised in Table 1.
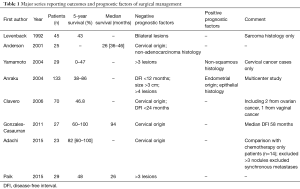
Full table
The case of uterine leiomyosarcomas
There is debate as to whether leiomyosarcomas of uterine origin and non-uterine origin may in fact be considered as similar entities and grouped together rather than per vicinal anatomy, with opposite evidence provided by different studies (20-22).
Uterine leiomyosarcomas are notoriously resistant to chemotherapy and prognosis is limited when lung metastases occur. This is reflected in a series from 2013, where in 192 patients with metastatic disease the presence of lung metastases was found to be a significantly negative prognostic factor. Those patients selected for and who survived extensive resection of the primary and lung metastasectomy had a more favourable outcome than the 5- and 10-year disease-specific survival of 30% and 15% respectively for the whole group. Notably all survivors at 10 years underwent complete surgical resection with disease limited to a single organ, most commonly the lung. Multiple lesions or dissemination to different organs were less likely to be resected, reflecting more aggressive biological behaviour of these sarcomas (11).
The case of benign metastasizing leiomyoma (BML)
BML is a rare disease and is defined as the growth of tissue from benign uterine leiomyoma in distant organs, most commonly the lungs. Previous history of benign uterine myoma is almost always present and nodules usually result histologically identical to the primary uterine tumour (23). Metastases to organs other than lung have been described, as well as miliary, giant, cystic and cavitary forms (24-27). Less than 100 cases have been reported in literature.
Lesions are usually slow-growing, although symptoms such as cough and chest pain are not rarely reported (28,29). At least one case of death from respiratory failure due to massive hilar metastases and a case of potentially fatal haemoptysis have been described (30,31). Cases of malignant transformation have also been described (32,33). Surgical removal should therefore be considered depending on patient fitness and on the chances of complete resectability. Unresectable disease may be treated with hormone therapy with usually excellent long-term control, although cases not responding to medical treatment have not rarely been described (34).
Cases of BML diagnosed in patients with previous history of malignant cancers (renal, bone sarcoma, ovarian) have been reported (35-38), and as prognosis for treated BML is very good compared to metastatic cancer, surgical biopsy should be considered when previous history of uterine myoma is also present, justifying in this case even a very aggressive removal of all lesions (39).
Conclusions
The treatment of metastatic uterine cancer with lung metastasectomy remains controversial. The evidence for surgery is limited to highly selected cases with the best prognosis (40). Favourable prognostic factors include a longer DFI and a limited number of lung lesions that are completely resectable. However, the proof that these patients would have done as well without surgery is lacking and in this rare disease a randomised controlled trial seems unlikely. The question remains if “doing the patient no harm” is a sufficient indication for an operation. Metastasectomy may be considered only with the patient fully understanding the doubts that this practice inevitably implies (41).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Internullo E, Cassivi SD, Van Raemdonck D, et al. Pulmonary metastasectomy: a survey of current practice amongst members of the European Society of Thoracic Surgeons. J Thorac Oncol 2008;3:1257-66. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Treasure T, Milošević M, Fiorentino F, et al. Pulmonary metastasectomy: what is the practice and where is the evidence for effectiveness? Thorax 2014;69:946-9. [Crossref] [PubMed]
- Hacker NF, Rao A. Surgical management of lung, liver and brain metastases from gynecological cancers: a literature review. Gynecol Oncol Res Pract 2016;3:7. [Crossref] [PubMed]
- Anraku M, Yokoi K, Nakagawa K, et al. Pulmonary metastases from uterine malignancies: results of surgical resection in 133 patients. J Thorac Cardiovasc Surg 2004;127:1107-12. [Crossref] [PubMed]
- Bouros D, Papadakis K, Siafakas N, et al. Patterns of pulmonary metastasis from uterine cancer. Oncology 1996;53:360-3. [Crossref] [PubMed]
- González Casaurrán G, Simón Adiego C, Peñalver Pascual R, et al. Surgery of female genital tract tumour lung metastases. Arch Bronconeumol 2011;47:134-7. [Crossref] [PubMed]
- Paik ES, Yoon A, Lee YY, et al. Pulmonary metastasectomy in uterine malignancy: outcomes and prognostic factors. J Gynecol Oncol 2015;26:270-6. [Crossref] [PubMed]
- Adachi M, Mizuno M, Mitsui H, et al. The prognostic impact of pulmonary metastasectomy in recurrent gynecologic cancers: a retrospective single-institution study. Nagoya J Med Sci 2015;77:363-72. [PubMed]
- Yamamoto K, Yoshikawa H, Shiromizu K, et al. Pulmonary metastasectomy for uterine cervical cancer: a multivariate analysis. Ann Thorac Surg 2004;77:1179-82. [Crossref] [PubMed]
- Lusby K, Savannah KB, Demicco EG, et al. Uterine leiomyosarcoma management, outcome, and associated molecular biomarkers: a single institution's experience. Ann Surg Oncol 2013;20:2364-72. [Crossref] [PubMed]
- Torek F. Removal of metastatic carcinoma of the lung and mediastinum. Arch Surg 1930;21:1416-24. [Crossref]
- Anderson TM, McMahon JJ, Nwogu CE, et al. Pulmonary resection in metastatic uterine and cervical malignancies. Gynecol Oncol 2001;83:472-6. [Crossref] [PubMed]
- Levenback C, Rubin SC, McCormack PM, et al. Resection of pulmonary metastases from uterine sarcomas. Gynecol Oncol 1992;45:202-5. [Crossref] [PubMed]
- Dowdy SC, Mariani A, Bakkum JN, et al. Treatment of pulmonary recurrences in patients with endometrial cancer. Gynecol Oncol 2007;107:242-7. [Crossref] [PubMed]
- Bouros D, Papadakis K, Siafakas N, et al. Natural history of patients with pulmonary metastases from uterine cancer. Cancer 1996;78:441-7. [Crossref] [PubMed]
- Otsuka I, Ono I, Akamatsu H, et al. Pulmonary metastasis from endometrial carcinoma. Int J Gynecol Cancer 2002;12:208-13. [Crossref] [PubMed]
- Shiromizu K, Kasamatsu T, Takahashi M, et al. A clinicopathological study of postoperative pulmonary metastasis of uterine cervical carcinomas. J Obstet Gynaecol Res 1999;25:245-9. [Crossref] [PubMed]
- Clavero JM, Deschamps C, Cassivi SD, et al. Gynecologic cancers: factors affecting survival after pulmonary metastasectomy. Ann Thorac Surg 2006;81:2004-7. [Crossref] [PubMed]
- Farid M, Ong WS, Tan MH, et al. The influence of primary site on outcomes in leiomyosarcoma: a review of clinicopathologic differences between uterine and extrauterine disease. Am J Clin Oncol 2013;36:368-74. [Crossref] [PubMed]
- Moinfar F, Azodi M, Tavassoli FA. Uterine sarcomas. Pathology 2007;39:55-71. [Crossref] [PubMed]
- Lamm W, Natter C, Schur S, et al. Distinctive outcome in patients with non-uterine and uterine leiomyosarcoma. BMC Cancer 2014;14:981. [Crossref] [PubMed]
- Kayser K, Zink S, Schneider T, et al. Benign metastasizing leiomyoma of the uterus: documentation of clinical, immunohistochemical and lectin-histochemical data of ten cases. Virchows Arch 2000;437:284-92. [Crossref] [PubMed]
- Abramson S, Gilkeson RC, Goldstein JD, et al. Benign metastasizing leiomyoma: clinical, imaging, and pathologic correlation. AJR Am J Roentgenol 2001;176:1409-13. [Crossref] [PubMed]
- Hoetzenecker K, Ankersmit HJ, Aigner C, et al. Consequences of a wait-and-see strategy for benign metastasizing leiomyomatosis of the lung. Ann Thorac Surg 2009;87:613-4. [Crossref] [PubMed]
- Miller J, Shoni M, Siegert C, et al. Benign metastasizing leiomyomas to the lungs: an institutional case series and a review of the recent literature. Ann Thorac Surg 2016;101:253-8. [Crossref] [PubMed]
- Lee SR, Choi YI, Lee SJ, et al. Multiple cavitating pulmonary nodules: rare manifestation of benign metastatic leiomyoma. J Thorac Dis 2017;9:E1-5. [Crossref] [PubMed]
- Grafino M, Ferreira L, Telo L, et al. A rare cause of miliary pattern and respiratory failure–Benign metastasizing leiomyoma. Rev Port Pneumol (2006) 2016;22:296-7.
- Pastré J, Juvin K, Grand B, et al. Pulmonary benign metastasizing leiomyoma presented as acute respiratory distress. Respirol Case Rep 2017;5:e00216. [Crossref] [PubMed]
- Rege AS, Snyder JA, Scott WJ. Benign metastasizing leiomyoma: a rare cause of multiple pulmonary nodules. Ann Thorac Surg 2012;93:e149-51. [Crossref] [PubMed]
- Miyazaki M, Nakayama A, Noda D, et al. Difficulty in complete transarterial embolization for pulmonary benign metastasizing leiomyoma with massive hemoptysis. Jpn J Radiol 2014;32:53-7. [Crossref] [PubMed]
- Song KS, Keum DY, Hwang IS. Malignant transformation of pulmonary benign metastasizing leiomyoma. Korean J Thorac Cardiovasc Surg 2017;50:59-63. [Crossref] [PubMed]
- Ogawa M, Hara M, Ozawa Y, et al. Benign metastasizing leiomyoma of the lung with malignant transformation mimicking mediastinal tumor. Clin Imaging 2011;35:401-4. [Crossref] [PubMed]
- Chen S, Liu RM, Li T. Pulmonary benign metastasizing leiomyoma: a case report and literature review. J Thorac Dis 2014;6:E92-8. [PubMed]
- Osadchy A, Zehavi T, Zissin R. Pulmonary benign metastasising leiomyomas presenting as fluid-containing masses on CT in a patient with two unrelated malignancies. Br J Radiol 2005;78:639-41. [Crossref] [PubMed]
- Del Real-Romo ZJ, Montero-Cantú C, Villegas-Cabello O, et al. Incidental benign metastasizing leiomyoma in a patient with bone sarcoma: a case report. Case Rep Surg 2014;2014:439061. [Crossref] [PubMed]
- Chen YY, Wu ST, Hsu HH, et al. Pulmonary leiomyomas in a patient with bilateral renal cell cancer mimicking pulmonary metastases. Clin Imaging 2014;38:330-2. [Crossref] [PubMed]
- Gan MF, Lu HS. An undescribed coexistence of benign metastasizing leiomyoma in the lung with serous borderline tumor of the ovary. Eur J Gynaecol Oncol 2013;34:193-5. [PubMed]
- Ottlakan A, Borda B, Lazar G, et al. Treatment decision based on the biological behavior of pulmonary benign metastasizing leiomyoma. J Thorac Dis 2016;8:E672-6. [Crossref] [PubMed]
- Åberg T, Treasure T. Analysis of pulmonary metastasis as an indication for operation: an evidence-based approach. Eur J Cardiothorac Surg 2016;50:792-8. [Crossref] [PubMed]
- Dunning J. A view of the pulmonary metastasectomy in colorectal cancer (PulMiCC) trial from the coalface. Eur J Cardiothorac Surg 2016;50:798-9. [Crossref] [PubMed]


