Percutaneous coronary intervention followed by minimally invasive valve surgery compared with median sternotomy coronary artery bypass graft and valve surgery in patients with prior cardiac surgery
Introduction
The incidence of re-operative valve surgery is increasing owing to improved surgical techniques, a greater survival rate after cardiac surgery, and an increased aging population (1). In patients with a prior sternotomy, valvular surgery via repeat median sternotomy (ST) carries significant risks, including increased bleeding, possible injury to cardiac structures or patent grafts, and a higher operative mortality (2-5). By utilizing a minimally invasive approach in patients requiring re-operative valve surgery, one may avoid a repeat ST, the need for dissection of pericardial adhesions, and reduce surgical trauma. Indeed, minimally invasive valve surgery (MIVS) in patients undergoing re-operative surgery is associated with less bleeding, reduced blood transfusions, absence of deep sternal wound infections, shorter hospital length of stay, and improved post-operative outcomes (6-8).
In patients with a history of prior cardiac surgery requiring re-operative coronary artery revascularization and valve surgery (CABG + valve), performing percutaneous coronary intervention (PCI) for revascularization permits the use of minimally invasive surgical techniques (a “hybrid” approach), and avoids performing a combined CABG + valve surgery via a repeat ST (9-16). Hybrid PCI + MIVS has been demonstrated to be associated with less bleeding, lower resource utilization, less composite complications, and at least comparable clinical outcomes, when compared with primary CABG + valve surgery (12,15,16). We hypothesized that in patients requiring re-operative CABG + valve surgery, PCI + MIVS may offer an alternative to the standard CABG + valve surgery via ST. Herein, we compared the outcomes of patients with a history of cardiac surgery performed via ST who underwent re-operative PCI + MIVS versus CABG + valve surgery via repeat ST.
Methods
This study was approved by the Institutional Review Board at Mount Sinai Medical Center, Miami Beach, Florida. A retrospective review of our Institutional Society of Thoracic Surgeons (STS) database was performed to identify patients with a history of cardiac surgery who subsequently presented with coronary artery and valvular disease requiring repeat surgical intervention between February 2009 and April 2014. The outcomes of those who underwent PCI + MIVS were compared with those who underwent CABG + valve surgery via ST.
The definitions and variables selected were based on the STS database definitions. The variables analyzed were operative mortality, as well as, postoperative complications. Operative mortality was defined as death within 30 days of surgery, or at any time after the operation if the patient was not discharged from the hospital alive. Operative times, as well as intensive care unit and total hospital lengths of stay, were also assessed. Patients undergoing emergency surgery, those with endocarditis, and those undergoing a concomitant procedure of the aorta were excluded. All surviving patients were evaluated in the outpatient setting 30 days after surgery by the Heart Valve Team. Follow-up data concerning survival and major adverse cardiac and cerebrovascular events (MACCE) were assessed by searching local electronic health records, cardiology office follow-up visits, and a telephone follow-up survey every six months using a questionnaire approved by the Institutional Review Board. Vital status for all patients was also assessed with the Social Security Death Index.
Patient selection and technique for PCI + MIVS
In all patients, the coronary and valvular lesions were documented by diagnostic catheterization and echocardiography. The decision to proceed with a strategy of PCI + MIVS was made by the Heart Valve Team, with consideration of the coronary anatomy and feasibility of PCI, co-morbidities and surgical risk factors, and patient preference. A loading dose of 600 mg of clopidogrel and 325 mg of aspirin was administered at the time of stent placement, followed by clopidogrel 75 mg daily and aspirin 81 to 325 mg daily. Patients continued their anti-platelet therapy up to the day of MIVS and this was resumed on day one or two post-operatively.
Our MIVS approach has been described previously in detail, with a brief summary provided herein (17). For aortic valve procedures, a 5–6 cm right transverse skin incision was made 1 cm lateral to the sternum over the 2nd to 3rd intercostal space. The 2nd or 3rd costochondral cartilage was transected for the surgery, and then re-attached at the conclusion of the procedure. For combined aortic and mitral valve procedures, a 6–7 cm incision is performed over the 4th intercostal space starting at the mid-clavicular line. In patient undergoing mitral valve surgery, a 5–6 cm skin incision was made in the 4–5th intercostal space lateral to the anterior axillary line. The mitral valve was accessed through Waterston’s groove, with the typical left atriotomy. Finally, in combined mitral and tricuspid valve surgery, a 6 cm incision was made in the right 4th to 5th intercostal space lateral to the anterior axillary line.
Statistical methods
All continuous variables were expressed as the median and interquartile range (IQR, or 25%–75%) or mean ± 1 standard deviation (SD). Continuous variables with normal distribution were analyzed using Student’s t-test. The Mann-Whitney U-test was utilized to compare those variables with nonparametric distributions. All dichotomous variables were compared using chi-square analysis. A two-tailed P value of <0.05 was considered statistically significant. The statistical analyses were conducted using Statistical Package for Social Sciences, version 21 (SPSS Inc., Chicago, IL, USA).
Results
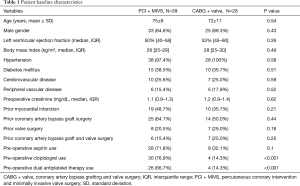
There were 67 patients identified with a history of prior cardiac surgery that required coronary artery revascularization and valve surgery, of which 39 underwent PCI + MIVS and 28 underwent CABG + valve surgery via repeat ST. There were 33 (84.6%) men in the PCI + MIVS group and 25 (89.3%) in the CABG + valve group (P=0.43), with a mean age of 75±9 and 72±11 years (P=0.54), respectively. There were no significant differences in the types of previous cardiac surgery between the two groups. In the PCI + MIVS group, this consisted of CABG in 25 (64.1%) patients, valve surgery in 8 (20.5%), and CABG + valve surgery in 6 (15.4%). In the CABG + valve group, there were 14 (50%) with prior CABG, 7 (25%) with prior valve surgery, and 7 (25%) with prior CABG + valve surgery. The baseline characteristics were similar between both groups, with the exception of a higher pre-operative use of clopidogrel and dual antiplatelet therapy in the PCI + MIVS group compared with the CABG + valve group, being 30 (76.9%) vs. 4 (14.3%), (P<0.001), and 26 (66.7%) vs. 4 (14.3%), (P<0.001), respectively (Table 1).
Full table
In the PCI + MIVS group, 20 (51.2%) of the patients had their PCI at another hospital, and were referred to our Medical Center for their valve surgery. There were 35 (89.7%) patients who had 1-vessel, and 4 (10.3%) had 2-vessel PCI, with a median of 1 stent (IQR, 1–2) placed (Table 2). The median time from PCI to MIVS was 43 (IQR, 19–71) days. In this group, single valve surgery was performed in 30 (76.9%) patients, consisting of 16 (41%) aortic valve replacements, 7 (17.9%) mitral valve replacements, 7 (17.9%) mitral valve repairs, and 9 (23.1%) double valve operations. Adequate exposure of the surgical field was obtained in all the minimally invasive operations, with no patients requiring conversion to a full sternotomy. In patients undergoing CABG + valve surgery, the prevalence of 1-, 2-, and 3-vessel coronary artery disease was 6 (21.4%), 4 (14.3%),and 18 (64.3%). Single valve surgery was performed in 21 (83.3%) patients, consisting of 16 (57%) aortic valve replacements, 3 (10.7%) mitral valve replacements, 2 (7.1%) mitral valve repairs, and 7 (25%) double valve operations. There was no significant difference between the two groups in regards to the type of valve surgery performed (Table 3).

Full table
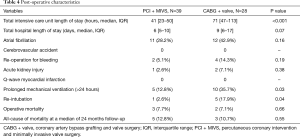
The median aortic cross-clamp and cardiopulmonary bypass times were 94 (IQR, 77–122) and 128 minutes (IQR, 100–158) for the PCI + MIVS group, versus 131 (IQR, 105–152) and 190 minutes (IQR, 146–219) for the CABG + valve group (P=0.001 and <0.001, respectively). The mean number of packed red blood cells units transfused intra-operatively were significantly lower in the PCI + MIVS group, being 1.3±1.5 vs. 3.8±3.1 units (P<0.001) (Table 3). The median intensive care unit length of stay was 41 hours (IQR, 23–50) vs. 71 (IQR, 47–113) for the PCI + MIVS and CABG + valve group (P<0.001), with a median total hospital length of stay of 6 days (IQR, 5–10) and 9 (IQR, 6–17), respectively (P=0.07). Additionally, patients who underwent PCI + MIVS had a lower prevalence of prolonged mechanical ventilation and re-intubation, which occurred in 5 (12.8%) and 1 (2.6%) patients, respectively, as compared with the CABG + valve group, in which there were 10 (35.7%) and 5 (17.9%) occurrences (P=0.03 and P=0.04, respectively). The operative mortality was 3 (7.7%) and 2 (7.1%) in the PCI + MIVS and CABG + valve group, respectively (P=0.66), and at a median follow-up of 24 months (IQR, 12–37), the all-cause mortality was 5 (12.8%) for the PCI + MIVS group, and 3 (10.7%) in the CABG + valve group (P=0.55) (Table 4).

Full table
Full table
Discussion
In patients with prior sternotomy requiring cardiac valve surgery, sternal re-entry carries significant risk of peri-operative complications, with operative mortality rates as high as 17% for isolated cardiac valve surgery (18,19). Injury to vascular structures or coronary artery grafts is a major concern, and mediastinal scarring and adhesions tend to make the intervention technically difficult. In patients requiring CABG + valve surgery, re-operation is even more challenging. In the present study, a strategy of hybrid PCI + MIVS when compared with CABG + valve surgery was associated with: (I) less need for intra-operative blood transfusions, despite a much higher prevalence of pre-operative clopidogrel or dual antiplatelet therapy usage; (II) a reduction in prolonged mechanical ventilation and re-intubation; (III) a faster post-operative recovery, as evidenced by a shorter intensive care unit length of stay; and, (IV) comparable operative mortality and early follow-up survival rates.
In general, MIVS is associated with longer operative times when compared with ST (20,21). However, by performing PCI to treat the coronary artery disease, one obviates the necessity of performing concomitant CABG at the time of surgery, significantly reducing the complexity of the surgery and shortening the operative times, which was noted in our study when compared with repeat ST. The less traumatic nature of MIVS and reduced operative times likely conferred lower transfusion requirements, despite more patients being on dual anti-platelet therapy. Although, our study was not powered to detect a statistically significant difference, shorter operative times and less blood product use during cardiac surgery are associated with fewer infections, and a lower morbidity and mortality (22,23).
After a median sternotomy, there are decreases in forced vital capacity, expiratory volume in the first second of forced expiration, peak expiratory flow rate, and maximum voluntary ventilation (24). The respiratory function is further impaired when there is harvesting of the internal mammary artery which causes a reduced blood supply to the intercostal muscles, which may decrease the forces of respiration with a corresponding decrease in pulmonary mechanics (25). Both of these issues, associated with ST and internal mammary harvesting, are typically avoided by the use of a PCI + MIVS approach. The present study confirmed this, demonstrating a significantly lower incidence of prolonged ventilation and re-intubation, leading to shorter intensive care unit length of stay with the PCI + MIVS approach, when compared with ST. These benefits of reduced ventilation times, and shorter intensive care unit length of stay, have been consistently demonstrated in patients with a previous ST who underwent a re-operative valve surgery via a minimally invasive approach (6-8).
A potential limitation of MIVS in patients undergoing re-operative valve surgery is the ability to obtain adequate exposure of the surgical field. In circumstances when adequate exposure cannot be obtained, the surgery may need to be converted to ST. The conversion rate of a MIVS to ST is approximately 2.6% to 4.0% (26). Obtaining appropriate exposure was not found to be a problem in our cohort, and none of the patients who underwent MIVS needed to be converted to ST.
The present study is subject to the limitations inherent to a single-center retrospective study. Firstly, its retrospective nature, and the selection of patients for PCI + MIVS based on the coronary anatomy confers a significant treatment selection bias. Secondly, the cohort consisted of a heterogeneous group of patients undergoing single vessel or multi-vessel PCI, and receiving different types of re-operative valve surgery. Patients undergoing PCI + MIVS had a greater prevalence of 1-vessel disease, while the CABG + valve group had more 3-vessel disease, introducing an uncontrollable confounder given the preference of CABG in this group of patients (27). Nevertheless, there were similar rates of left main and proximal left anterior coronary artery disease between the groups, suggesting a similar distribution of higher risk coronary lesions. Thirdly, follow-up outcomes were limited to all-cause mortality, with no data available on the development of target vessel revascularization or myocardial infarction, which are important determinants of long-term PCI success. Finally, the study sample size was small, which limited the statistical power and may underestimate differences in demographics and clinical variables and outcomes.
In conclusion, the present study suggests that in patients with prior cardiac surgery requiring re-operation for concomitant coronary artery and valvular disease, a hybrid approach of PCI + MIVS for single or double valve surgery is associated with shorter operative times, a lower blood transfusion requirement, and a reduction in prolonged mechanical ventilation with similar short-term and follow-up survival when compared with CABG + valve via ST. However, our sample size is small, and heterogeneous, limiting the conclusions that can be drawn. Our results are best interpreted as providing evidence for the safety and feasibility of MIVS via a right thoracotomy as an acceptable alternative to conventional median repeat sternotomy, for patients with previous cardiac surgery that require a re-operation for coronary artery and valvular disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board at Mount Sinai Medical Center, Miami Beach, Florida.
References
- Brown JM, O’Brien SM, Wu C, et al. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in The Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg 2009;137:82-90. [Crossref] [PubMed]
- Edwards FH, Peterson ED, Coombs LP, et al. Prediction of operative mortality after valve replacement surgery. J Am Coll Cardiol 2001;37:885-92. [Crossref] [PubMed]
- Follis FM, Pett SB, Miller KB, et al. Catastrophic hemorrhage on sternal reentry: Still a dreaded complication? Ann Thorac Surg 1999;68:2215-19. [Crossref] [PubMed]
- Toker ME, Eren E, Guler M, et al. Second and third cardiac valve re-operations: Factors influencing death and long-term survival. Tex Heart Inst J 2009;36:557-62. [PubMed]
- Balsam LB, Grossi EA, Greenhouse DG, et al. Re-operative valve surgery in the elderly: Predictors of risk and long-term survival. Ann Thorac Surg 2010;90:1195-200. [Crossref] [PubMed]
- Murzi M, Solinas M, Glauber M. Is a minimally invasive approach for re-operative mitral valve surgery superior to standard resternotomy? Interact CardioVasc Thorac Surg 2009;9:327-32. [Crossref] [PubMed]
- Mihos CG, Santana O, Lamas GA, et al. Outcomes of right mini-thoracotomy mitral valve surgery in patients with previous sternotomy. Ann Thorac Surg 2011;91:1824-7. [Crossref] [PubMed]
- Pineda AM, Santana O, Lamas GA, et al. Is a minimally invasive approach for re-operative aortic valve replacement superior to standard full resternotomy? Interact Cardiovasc Thorac Surg 2012;15:248-52. [Crossref] [PubMed]
- Byrne JG, Leacche M, Unic D, et al. Staged initial percutaneous coronary intervention followed by valve surgery ("hybrid approach") for patients with complex coronary and valve disease. J Am Coll Cardiol 2005;45:14-18. [Crossref] [PubMed]
- Brinster DR, Byrne M, Rogers CD, et al. Effectiveness of same day percutaneous coronary intervention followed by minimally invasive aortic valve replacement for aortic stenosis and moderate coronary disease ("hybrid approach"). Am J Cardiol 2006;98:1501-3. [Crossref] [PubMed]
- Umakanthan R, Leacche M, Petracek MR, et al. Combined PCI and minimally invasive heart valve surgery for high-risk patients. Curr Treat Options Cardiovasc Med 2009;11:492-98. [Crossref] [PubMed]
- Santana O, Funk M, Zamora C, et al. Staged percutaneous coronary intervention and minimally invasive valve surgery: Results of a hybrid approach to concomitant coronary and valvular disease. J Thorac Cardiovasc Surg 2012;144:634-9. [Crossref] [PubMed]
- Santana O, Pineda AM, Cortes-Bergoderi M, et al. Hybrid approach of percutaneous coronary intervention followed by minimally invasive valve operations. Ann Thorac Surg 2014;97:2049-55. [Crossref] [PubMed]
- George I, Nazif TM, Kalesan B, et al. Feasibility and early safety of single-stage hybrid coronary intervention and valvular cardiac surgery. Ann Thorac Surg 2015;99:2032-7. [Crossref] [PubMed]
- Mihos CG, Santana O, Pineda AM, et al. Percutaneous coronary intervention followed by minimally invasive mitral valve surgery in ischemic mitral regurgitation. Innovations (Phila) 2015;10:394-7. [Crossref] [PubMed]
- Santana O, Singla S, Mihos CG, et al. Outcomes of the hybrid approach of combined percutaneous coronary revascularization and minimally invasive valve surgery in patients with concomitant coronary and valvular heart disease. Innovations (Phila) 2017;12:4-8. [Crossref] [PubMed]
- Santana O, Reyna J, Grana R, et al. Outcomes of minimally invasive valve surgery versus standard sternotomy in obese patients undergoing isolated valve surgery. Ann Thorac Surg 2011;91:406-10. [Crossref] [PubMed]
- Borger MA, Yau TM, Rao V, et al. Reoperative mitral valve replacement: importance of preservation of the subvalvular apparatus. Ann Thorac Surg 2002;74:1482-7. [Crossref] [PubMed]
- Odell JA, Mullany CJ, Schaff HV, et al. Aortic valve replacement after previous coronary artery bypass grafting. Ann Thorac Surg 1996;62:1424-30. [Crossref] [PubMed]
- Schmitto JD, Mokashi SA, Cohn LH. Minimally-invasive valve surgery. J Am Coll Cardiol 2010;56:455-62. [Crossref] [PubMed]
- Cheng DC, Martin J, Lal A, et al. Minimally invasive versus conventional open mitral valve surgery: a meta-analysis and systematic review. Innovations (Phila) 2011;6:84-103. [Crossref] [PubMed]
- Murphy GJ, Reeves BC, Rogers CA, et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation 2007;116:2544-52. [Crossref] [PubMed]
- Kumar AB, Suneja M, Bayman EO, et al. Association between postoperative acute kidney injury and duration of cardiopulmonary bypass: a meta-analysis. J Cardiothorac Vasc Anesth 2012;26:64-9. [Crossref] [PubMed]
- Weissman C. Pulmonary function after cardiac and thoracic surgery. Anesth Analg 1999;88:1272-9. [Crossref] [PubMed]
- Berrizbeitia LD, Tessler S, Jacobowitz IJ, et al. Effect of sternotomy and coronary bypass surgery on postoperative pulmonary mechanics. Chest 1989;96:873-6. [Crossref] [PubMed]
- Tabata M, Umakanthan R, Khalpey Z, et al. Conversion to full sternotomy during minimal-access cardiac surgery: reasons and results during a 9.5 year experience. J Thorac Cardiovasc Surg 2007;134:165-9. [Crossref] [PubMed]
- Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol 2011;58:e123-210. [Crossref] [PubMed]


