In the era of ultrasound technology, could conventional trans-bronchial needle aspiration still play a role in lung cancer mediastinal staging?
Introduction
Accurate staging of lung cancer with preoperative detection of mediastinal spread is critical for planning optimal management, including resection with curative intent (1,2). Conventional-trans-bronchial needle aspiration biopsy (C-TBNA) has been an available procedure for sampling lung tissue and/or mediastinal adenopathy for almost 3 decades with a sensitivity ranging from 39% to 78% (3-5). Despite its proven efficacy, it remained underutilized in clinical practice for potential reasons as the risk of puncturing vessels, the fear of damaging bronchoscopy, and poor specimen preparation. However, the only great flaw of TBNA was its “blindness” (6). The introduction in the last decade of ultrasound technology integrated to bronchoscopy has revolutionized the strategy of mediastinal sampling and stimulated a resurgence in training and performance of TBNA. EBUS allows to have a real time guidance of the sampling procedure with a sensitivity ranging from 83% to 94% in mediastinal staging, as reported in several metanalysis (7-11). Thus, the recent American College of Chest Physician (ACCP) guidelines (12) recommend a fine-needle aspiration (FNA) with endobronchial (EBUS) or endoscopic (EUS)—or even combined (EBUS/EUS)—ultrasound guidance as first test in non small cell lung cancer (NSCLC) patients with high suspicion of mediastinal lymph nodes (LN) involvement. Despite all, it is currently a disputed subject whether all mediastinal LNs should be sampled by endobronchial ultrasounds transbronchial needle aspiration (EBUS-TBNA) considering the high cost and the limited availability of this procedure.
Thus, the aim of the present paper was to evaluate the feasibility of a combined strategy including C-TBNA and EBUS-TBNA for sampling mediastinal adenopathies in patients with lung cancer in order to determinate whether in the era of EBUS technology C-TBNA could continue to play a role in the setting of mediastinal staging.
Methods
Study design
It was a retrospective multicenter study performed at Thoracic Surgery Unit of Second University of Naples, Naples, Italy and at Endoscopic Unit of Mauro Scarlato Hospital, Scafati, Italy. All consecutive patients with lung cancer and undergoing TBNA for mediastinal staging between January 2014 and July 2016 were eligible. Criteria inclusion were (I) age >18 years old, and (II) mediastinal involvement on positron emission tomography-computed tomography (PET-CT) scan in stations accessible by TBNA (paratracheal and subcarinal). Patients were excluded in case of lack of definitive histological diagnosis (i.e., negative C-TBNA not confirmed by other invasive exams as EBUS-TBNA and/or mediastinoscopy).
Data were recorded in a prospective database and then retrospectively analyzed. The diagnostic yield of C-TBNA was evaluated in relation to size and site of target lesion in order to support the hypothesis that C-TBNA could still play a role in mediastinal staging of lung cancer (hypothesis of the study). All patients gave a signed written informed consent for all invasive procedures and surgery and were aware that the data could be used anonymously for scientific purpose only.
Study population
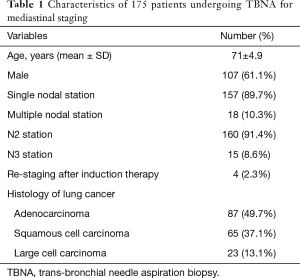
Overall, 187 consecutive patients with NSCLC and radiological mediastinal adenopathies underwent TBNA in the study period. All patients received a contrast enhanced whole body PET/CT. Mediastinal LNs were considered potentially metastatic if the short-axis diameter was >10 mm and/or the standardized uptake value (SUV) was >2.5 or had an uptake greater than the background activity of the mediastinum (13,14). TBNA biopsy was subcategorized and analyzed for diagnostic success based on the LN station as paratracheal (stations 2R, 4R, 2L, 4L) and subcarinal (station 7) according to the Mountain-Dresler classification (15) and on LN size (measure in mm).
In all cases, C-TBNA was performed as first diagnostic invasive test. In patients with multi-adenopathy stations, the station with higher SUV value and/or having larger size was biopsied. Positive C-TBNA results were accepted as accurate and patients were excluded from surgery and underwent chemotherapy and/or radiotherapy as indicated. All negative C-TBNA results were always followed by EBUS-TBNA to clarify if they were false negative or a true negative results; negative EBUS-TBNA results were then confirmed by surgery or in patients with a high pre-test clinical probability of malignancy by surgical biopsy through mediastinoscopy. In all clinical N0 patients undergoing surgery, pulmonary resection was associated with complete LN dissection in order to confirm the nature of the adenopathies.
Procedures
C-TBNA
It was performed using disposable 21-gauge cytological needles by the same experienced thoracic surgeon (AF) and using the same standard flexible bronchoscope (models BF-T160; Olympus; Tokyo, Japan). The exams were performed in a dedicated bronchoscopic room generally under local anaesthetic and only in select cases (i.e., anxious patients) under sedation with midazolam. In the same setting of diagnostic bronchoscopy, to avoid needle contamination, TBNA was initially performed and bronchoalveolar lavage, brushing and biopsy were then carried out as clinically indicated. The needle was inserted using orientation with axial CT sections and the number of needle passes ranged from 3 to 5. On removal the needle, the aspirate was smeared onto microscopic slides. Rapid on-site cytology evaluation (ROSE) was not available. Cytological specimens were reported as either positive for malignancy, or negative for malignancy or an inadequate specimen when lymphocytes were not found in the specimen.
EBUS-TBNA
It was performed by the same experienced bronchoscopist (CS). All exams were performed under sedation and spontaneous breathing and ROSE was available in the most of cases. The EBUS device (model BF-UC180F; Olympus Corp., Tokyo, Japan) was introduced orally. After ultrasound identification of the adenopathy, a 22-gauge aspiration needle (model NA-201SX-402; Olympus Corp.) was introduced into the working channel of the device and a puncture was performed through the tracheobronchial wall. The material collected was prepared on slides and immediately evaluated by a cytopathologist to determinate whether it was adequate for a preliminary diagnosis. If the preliminary interpretation was negative, inadequate, or inconclusive, additional passes were made with the needle into the same or a different lymph node at the discretion of the physician. In the last puncture, needle was washed in a phosphate-buffered sucrose (PBS) solution, including the remaining material in the needle with more probability of blood clot, to perform subsequently a cytoblock for any additional immunohistochemical or molecular marker studies.
Mediastinoscopy
Video-mediastinoscopy was performed under general anesthesia with oro-tracheal intubation. Stations 2R, 4R, 2L, and 7 (highest part of the station) were biopsied.
Statistical analysis
The Kolmogorov-Smirnov test and graphic histograms were used to check the normality/skewness of continuous variables data in subgroups before further analysis, and appropriate statistical tests have been chosen accordingly. Data were summarized as mean and standard deviation (SD) for normally distributed continuous variables; median and inter quartile range (IQR) 25th–75th percentiles for skewness continuous variables or absolute number and percentage for categorical variables, as appropriate.
Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and diagnostic accuracy with confidence interval (CI) at 95% CI of the procedures was calculated in a standard manner. Mann-Whitney test, Chi-square test with Yates correction and Fisher exact test were used to evaluate the intergroup differences, as appropriate. A value of P<0.05 was considered statistically significant. MedCalc statistical software (Version 12.3, Broekstraat 52; Mariakerke, Belgium) was used for analysis.
Results
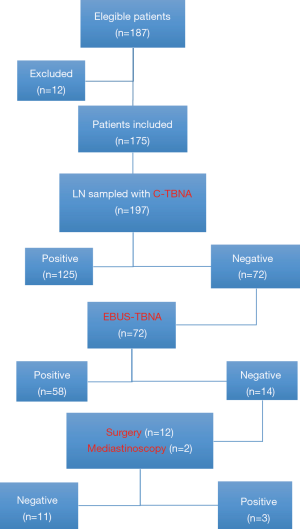
During the study period, 187 patients undergoing C-TBNA for mediastinal staging (n=183) or re-staging (n=4) following induction therapy. Of these, 12 patients were excluded due the lack of definitive histological diagnosis (i.e., negative C-TBNA and/or EBUS-TBNA negative results not confirmed by surgical biopsy or surgical exploration). Thus, 175 patients were included in the study for a total of 197 LNs sampled. The characteristics of our study population including demographic, clinical and pathological data are summarized in Table 1. C-TBNA was performed as first exam in all cases and resulted to be positive in 125 cases (excluded from surgery) and negative in 72 cases. EBUS-TBNA was performed in all negative C-TBNA cases (n=72) and was positive in 58 cases (excluded from surgery) and negative in 14 patients. Of these, 12 patients underwent surgery with resection of tumor and radical lymph adenectomy that showed no LN involvement (pN0) in all cases but one while the remaining two patients due to high suspicion of mediastinal involvement underwent mediastinoscopy that showed the presence of metastases in both cases. The flow chart of the study is summarized in Figure 1.
Full table
Diagnostic yield of C-TBNA
The disease prevalence was 94.42% (90.23–97.18%) since 186/197 patients had a mediastinal LN involvement. TBNA resulted to be positive in 125 cases, true negative in 11 cases and false negative in 61 cases. Sensitivity; specificity; PPV; NPV; and diagnostic accuracy values were 67.20% (59.95–73.90%); 100% (71.51–100%); 100% (97.09–100%); 15.28% (7.88–25.69%); and 69.04% (62.00–75.34%), respectively
Diagnostic yield of C-TBNA in relation to LN station
The results are summarized in Table 2. Among the 197 adenopathies sampled, 111/197 (56.35%) were located in paratracheal station and 86/197 (43.65%) in subcarinal station. The comparison between paratracheal versus subcarinal stations showed no significant difference regarding disease prevalence (94.59% vs. 94.19%), size (18±6.8 vs. 17.6±3.9; P=0.8) and SUV value (2.65±4.9 vs. 2.72±6.7; P=0.7). For paratracheal group, C-TBNA resulted to be positive in 85 cases, true negative in 6 cases and false negative in 20 cases. Sensitivity; specificity; PPV; NPV; and diagnostic accuracy values were 80.95% (72.13–87.96%); 100% (54.07–100%); 100% (95.75–100%); 23.08% (8.97–43.65%); and 81.98% (73.30–88.52%), respectively. For subcarinal group, C-TBNA resulted to be positive in 40 cases, true negative in 5 cases and false negative in 41 cases. Sensitivity; specificity; PPV; NPV; and diagnostic accuracy values were 49.38% (38.08–60.73%); 100% (47.82–100%); 100% (91.19–100%); 10.87% (3.62–23.57%); and 52.33% (41.34–63.12%). The sensitivity (P<0.001)and diagnostic accuracy (P<0.001) value of paratracheal group were significantly higher than those of subcarinal group.

Full table
Diagnostic yield of C-TBNA in relation to LN size
The results are summarized in Table 3. The mean size of all adenopathies was 18±7.4 mm. In 78/197 (39.59%) cases the size was <15 mm (mean 13±4.9) and in 119/197 (60.41%) cases ≥15 mm (mean 19±4.9). The comparison between two sub-groups showed no significant difference regarding disease prevalence, station and SUV value (2.7±2.9 vs. 2.64±6.5; P=0.8). For subgroups of LN <15 mm, C-TBNA was positive in 32 cases, true negative in 4 cases and false negative in 42 cases. Sensitivity, specificity, PPV, NPV and diagnostic accuracy values were 43.24% (31.77–55.28%), 100.00% (39.76–100%), 100% (89.11–100%), 8.70% (2.42–20.79%) and 46.15% (34.93–57.74%), respectively. For LN ≥15 mm, C-TBNA resulted to be positive in 93 cases, true negative in 7 cases, and false negative in 19 cases. Sensitivity, specificity, PPV, NPV and diagnostic accuracy values were 83.04% (74.78–89.47%), 100% (59.04–100%), 100.00% (96.11–100%), 26.92% (11.57–47.79%) and 84.03% (75.93–90.01%). The sensitivity (P<0.001) and diagnostic accuracy (P<0.001) value of large adenopathies (>15 mm in size) were significantly higher than of small adenopathies (<15 mm in size).

Full table
Discussion
TBNA was first described in the literature using a rigid bronchoscopy and rigid needle by Schieppati et al. in 1949. Thirty years later, flexible needles that could be used with flexible bronchoscopy were developed, and Wang et al. demonstrated the multiple applications of this technique using different types of needle (16). For more than 3 decades, C-TBNA has been considered a cheap and safe procedure for mediastinal staging in patients with lung cancer. C-TBNA is based off of anatomic land-marks and static CT correlation. Thus, the “blindness” of C-TBNA to see the LN to biopsy was the main limit for its widely use. In the last years, the development of health care technologies allowed to overcome the blindness of C-TBNA. EBUS is able to visualize and locate the target LN with ultrasound and then to perform the needle aspiration with real time ultrasound guidance. The most of studies (7-12) have been showed that EBUS-TBNA is more reliable and sensitive than C-TBNA for mediastinum staging and, in some of these EBUS-TBNA has superior results to mediastinoscopy, a fact that C-TBNA has never been able to claim. In line with these evidences (7-12), there is a growing mindset to abandon C-TBNA and that every mediastinal LNs aspiration should be EBUS guided. However, guidelines recommendations not only are depended on how well the procedure performs, but should also take many other factors into consideration, such as the invasiveness of the procedure, the availability of equipment and expert personnel, the risk and the cost. Compared to C-TBNA, EBUS-TBNA is a more complicated procedure that needs a huge training (at least 50 procedures per year to acquire and maintain the skills) available only in few high-volume centers (17). The acquisition and the running costs are other limiting factors for the widespread application of the EBUS-TBNA. If general anesthesia and ROSE are used for every case of EBUS-TBNA, the cost rises further. All these financial factors must be considered in choosing a diagnostic test, especially in this era of cost containment and reimbursement reduction. Thus, some authors (6,17,18) continue to promote the use of C-TBNA in mediastinal staging due to its low cost, ease of performance and training, and to consider EBUS-TBNA as a rescue modality for nondiagnostic C-TBNA. In fact, in some cases the continuous dependency and use of EBUS may be a passion and personal preference because of a lack of confidence and interest in conduction C-TBNA. Using general anesthesia can compensate for the inadequate skill of the bronchoscopist by improving the patient’s comfort and allowing more time for the procedure (19). Thus, in the present paper we aimed to evaluate whether C-TBNA should remain in the armamentarium of the physicians as a complementary procedure to EBUS-TBNA in diagnostic work-up of mediastinal staging.
In line with previous experiences (3-5), we found that the overall sensitivity of C-TBNA was 67% that increased in case of large adenopathies close to carina. In fact, the C-TBNA sensitivity for subcarinal station was significantly higher than for paratracheal stations (80.9% versus 49.4%, respectively, P<0.001) as well as C-TBNA sensitivity for large LN was significantly higher than for small LN (83.0% versus 43.2%, respectively, P<0.001). It is not surprising since small LNs are more difficult to biopsy especially if they are localized in “difficult station” as paratracheal station. Conversely, subcarinal station is usually considered an “easy station” to sample since the main carina is an important landmark that allows easy accessibility of the subcarinal adenopathy also with C-TBNA. Melloni et al. (3) reported that on 767 procedures for diagnosis and staging of lung cancer, the diagnostic yield of traditional TBNA was 81% and 42% for subcarinal and paratracheal lymph nodes, respectively. Herth et al. (4) randomized consecutive patients with mediastinal involvement to receive EBUS-TBNA or a C-TBNA. Patients with subcarinal lymph nodes were randomized and analyzed separately (group A) from all other stations (group B). Two hundred patients were examined (100 patients each in groups A and B). Half of the patients underwent EBUS-guided TBNA rather than conventional TBNA. In group A, the yield of conventional TBNA was 74% compared to 86% in the EBUS group (difference not significant). In group B, the overall yields were 58% and 84%, respectively. This difference was statistically highly significant (P<0.001). Phua et al. (20) assessed 35 C-TBNA procedures before and 45 of these procedures after intervention as well as 45 radial probe EBUS-TBNA and 50 linear EBUS-TBNA. The pre-intervention conventional TBNA yield was 43%, which improved to 82% after intervention. Although EBUS did not have an impact on TBNA yield (P=0.44) compared with the intervention (P=0.001), EBUS was useful for lymph nodes smaller than 2 cm (P<0.0001). Linear EBUS did not confer higher diagnostic accuracy than radial probe EBUS (P=0.47). Jiang et al. (21) in a recent prospective study including 253 patients undergoing both c-TBNA and EBUS-TBNA for diagnosis and staging of lung cancer, found no significant difference in the diagnostic yield of both methods among the 83/253 patients with a diagnosis of a malignancy. In addition, we found false-negative C-TBNA results for re-staging of mediastinal involvement (n=4), indirectly confirming previous experience that EBUS-TBNA remained the procedure of choice in case of re-staging.
In our clinical practice, we combined the use of C-TBNA and EBUS-TBNA for mediastinal staging according to an algorithm summarized in Figure 2. In patients with mediastinal adenopathies ≥15 mm, especially if located near to carina, we performed C-TBNA as first invasive procedure. In case of C-TBNA negative results, patient undergoes EBUS-TBNA; negative EBUS-TBNA results in patient with high pre-test clinical probability of malignancy will be then corroborated by mediastinoscopy. Conversely, EBUS-TBNA is the first choice for sampling all mediastinal adenopathies in patients who desire maximal assurance that successfully biopsy is achieved at the procedure and/or for sampling mediastinal adenopathies <15 mm far from the carina and/or re-staging of mediastinum after neoadjuvant therapy. All patients are always aware on the possibility of surgical biopsy in case of negative EBUS-TBNA results (7-12). This strategy is also endorsed by other authors (3,4,6,18-23) as the most practical and cost-effective. The combine use of C-TBNA and EBUS-TBNA allows the best use of the technical characteristics of both procedures. The use of C-TBNA as first diagnostic exam in selected cases could avoid more complex and invasive procedures reducing the cost of mediastinal staging as occurred in our 125 patients who resulted to be positive after C-TBNA and they did not undergo additional invasive procedures. On the other hand, EBUS-TBNA may overcome the problem of C-TBNA low yield in small adenopathies, far from the carina or for restaging after induction therapy as observed in our 58 cases with false-negative C-TBNA results. Obviously the widely acceptance of these recommendations depends on individual centers and bronchoscopist comfort level with TBNA (with or without EBUS).

Limitations
Our study presents the following limitations that should be taken in account before drawing definitive conclusions: (I) this study included only mediastinal LN. Yet, due to small number of lesions, we considered together adenopathies of station 2 and station 4, despite the difficult to sample could be different; (II) all positive C-TBNA results were considered as true positive since they were not corroborated by EBUS-TBNA as well as all positive EBUS-TBNA results were not corroborated by mediastinoscopy; (III) in this population, not all patients were potential surgical candidate, thus it explained the presence of large adenopathies (>15 mm in size) and multi-station adenopathies; (IV) ROSE was not available during C-TBNA with potential reduction of its diagnostic yield.
Conclusions
C-TBNA is a minimally invasive, safe and economical procedure that should remain in the armamentarium of every bronchoscopist also in center where EBUS is available. Our data support the combined use of C-TBNA and EBUS-TBNA as the most cost-effective strategy in the setting of mediastinal staging. C-TBNA performed before EBUS-TBNA is indicated for sampling large mediastinal LNs near to carina while EBUS-TBNA remains the first choice for puncturing small adenopathies far from carina and/or for mediastinal re-staging after induction therapy. However, future prospective randomized studies are necessary to confirm our results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors disclose that the present study does not require an ethics approval for the following reasons: (I) we apply diagnostic invasive exams as conventional-TBNA and EBUS-TBNA that are routinely used in clinical practice; (II) it is a retrospective study since all patients are treated according to the current clinical practice; (III) the study is designed at Thoracic Surgery Unit of Second University of Naples, a teaching hospital; thus, all patients are aware that their data could be used for scientific purpose only and they gave a written informed consent.
References
- Fiorelli A, Sagan D, Mackiewicz L, et al. Incidence, Risk Factors, and Analysis of Survival of Unexpected N2 Disease in Stage I Non-Small Cell Lung Cancer. Thorac Cardiovasc Surg 2015;63:558-67. [Crossref] [PubMed]
- Fiorelli A, Santini M. In lung cancer patients where a malignant pleural effusion is found at operation could resection ever still be justified? Interact Cardiovasc Thorac Surg 2013;17:407-12. [Crossref] [PubMed]
- Melloni G, Bandiera A, Muriana P, et al. Combined Use of TBNA and EBUS-TBNA in the Preoperative Staging of Lung Cancer Patients. J Bronchology Interv Pulmonol 2011;18:311-6. [Crossref] [PubMed]
- Herth F, Becker HD, Ernst A. Conventional vs endobronchial ultrasound-guided transbronchial needle aspiration: a randomized trial. Chest 2004;125:322-5. [Crossref] [PubMed]
- Fiorelli A, Rambaldi P, Vicidomini G, et al. Combined transbronchial needle aspiration and (99m)Tc-2-methoxy-isobutyl-isonitrile single photon emission computed tomography for diagnosing enlarged mediastinal lymph nodes. Arch Bronconeumol 2014;50:3-9. [PubMed]
- Trisolini R, Patelli M, Gasparini S. While waiting to buy a ferrari, do not leave your current car in the garage! Respiration 2010;79:452-3. [Crossref] [PubMed]
- Micames CG, McCrory DC, Pavey DA, et al. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: A systematic review and metaanalysis. Chest 2007;131:539-48. [Crossref] [PubMed]
- Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer 2009;45:1389-96. [Crossref] [PubMed]
- Adams K, Shah PL, Edmonds L, et al. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: systematic review and meta-analysis. Thorax 2009;64:757-62. [Crossref] [PubMed]
- Chandra S, Nehra M, Agarwal D, et al. Diagnostic accuracy of endobronchial ultrasound-guided transbronchial needle biopsy in mediastinal lymphadenopathy: a systematic review and meta-analysis. Respir Care 2012;57:384-91. [PubMed]
- Zhang R, Ying K, Shi L, et al. Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal lymph node staging of lung cancer: a meta-analysis. Eur J Cancer 2013;49:1860-7. [Crossref] [PubMed]
- Detterbeck FC, Jantz MA, Wallace M, et al. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:202S-20S.
- Fiorelli A, Vicidomini G, Laperuta P, et al. The role of Tc-99m-2-Methoxy-Isobutyl-Isonitrile Single Photon Emission Computed Tomography in visualizing anterior mediastinal tumor and differentiating histologic type of thymoma. Eur J Cardiothorac Surg 2011;40:136-42. [Crossref] [PubMed]
- Santini M, Fiorelli A, Vicidomini G, et al. F-18-2-fluoro-2-deoxyglucose positron emission tomography compared to technetium-99m hexakis-2-methoxyisobutyl isonitrile single photon emission chest tomography in the diagnosis of indeterminate lung lesions. Respiration 2010;80:524-33. [Crossref] [PubMed]
- Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest 1997;111:1718-23. [Crossref] [PubMed]
- Yang H, Zhang Y, Wang KP, et al. Transbronchial needle aspiration: development history, current status and future perspective. J Thorac Dis 2015;7:S279-86. [PubMed]
- Medford AR. An Interventional Pulmonologist’s Tool: Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (EBUS-TBNA) in Thoracic Disease—An Update. Current Respiratory Medicine Reviews 2012;8:396-408. [Crossref]
- Mehta AC, Wang KP. Teaching conventional transbronchial needle aspiration. A continuum. Ann Am Thorac Soc 2013;10:685-9. [Crossref] [PubMed]
- Kunst PW, Eberhardt R, Herth FJ. Combined EBUS Real Time TBNA and Conventional TBNA are the Most Cost-effective Means of Lymph Node Staging. J Bronchology Interv Pulmonol 2008;15:17-20.
- Phua GC, Rhee KJ, Koh M, et al. A strategy to improve the yield of transbronchial needle aspiration. Surg Endosc 2010;24:2105-9. [Crossref] [PubMed]
- Jiang J, Browning R, Lechtzin N, et al. TBNA with and without EBUS: a comparative efficacy study for the diagnosis and staging of lung cancer. J Thorac Dis 2014;6:416-20. [PubMed]
- Yarmus L, Feller-Kopman D, Browning R, et al. TBNA: should EBUS be used on all lymph node aspirations? J Bronchology Interv Pulmonol 2011;18:115-7. [Crossref] [PubMed]
- Fiorelli A, Raucci A, Cascone R, et al. Three-dimensional virtual bronchoscopy using a tablet computer to guide real-time transbronchial needle aspiration. Interact Cardiovasc Thorac Surg 2017;24:567-75. [PubMed]