Minimally invasive aortic valve replacement—where are we?
First described by Dr. Cribier and colleagues in 2002, transcatheter aortic valve replacement (TAVR) has established itself as a life-saving procedure for patients with severe symptomatic aortic stenosis (AS) (1). The PARTNER trial, which led the way in the US for studying TAVR as a treatment option for severe symptomatic AS, was conducted in two arms studying TAVR in patients deemed either inoperable or high risk for surgery. In the inoperable-surgical-risk arm, the superiority of Edwards SAPIEN balloon-expandable TAVR valve over medical treatment alone was demonstrated, while the high-surgical-risk arm showed non-inferior results for TAVR as compared to surgical aortic valve replacement (SAVR) (2,3). The CoreValve US Pivotal trial went one step further by showing the superiority of TAVR with a self-expandable CoreValve over SAVR in patients at high-surgical-risk for surgery (4). The 2014 American College of Cardiology/American Heart Association (ACC/AHA) guidelines for patients with valvular heart disease recognized the results of these clinical trials and included TAVR as a class I indication in patients with severe symptomatic AS considered prohibited risk of surgery; and as a reasonable alternative to SAVR in high-surgical-risk patients (5). Since then, TAVR has made rapid strides in both technological advances and operator experience, and has expanded its reach to the intermediate-surgical-risk patients (6,7). The PARTNER 2 trial compared outcomes of TAVR using the SAPIEN XT valve system against SAVR and showed similar overall outcomes in the two groups with respect to primary end points (death and stroke) in 2 years, although results favored TAVR in patients undergoing TAVR through the transfemoral route (6). In addition, TAVR also resulted in fewer bleeding complications; lower rates of acute kidney injury and new onset atrial fibrillation; shorter hospitalization and intensive care stay and larger aortic valve areas when compared to surgery. In comparison, it was noted to have a higher rate of paravalvular regurgitation (PVR), pacemaker implantations, and vascular complications.
Recently, Carnero-Alcázar et al. conducted a large meta-analysis comparing outcomes between TAVR and SAVR in patients with moderate to high surgical risk, irrespective of the type of valve and the route of access (8). This analysis comparing the two interventions included 45 studies and 20,224 patients. Studies included in this analysis had the Society of Thoracic Surgeons (STS) scores >4% or logistic European system for cardiac operative risk evaluation (EuroSCORE) >10%. In this meta-analysis, the rates of mortality and neurological complications were found to be comparable between the two interventions. The rates of bleeding and acute kidney injury were favorable for the TAVR group as compared to SAVR. Conversely, the rate of vascular complications, need for new pacemaker implantations, and residual aortic regurgitation (AR) favored the SAVR group. The investigators also compared hemodynamic performance of the two interventions and found TAVR to be superior due to lower trans-prosthetic gradients. They also conducted a subset analysis comparing TAVR against the sutureless AVR (SuAVR) and found SuAVR to have lower rates of early mortality and comparable rates of bleeding when compared to TAVR.
Although the rates of neurological complications for SAVR have been relatively stable over the years, the rates of neurological complications with TAVR have steadily declined since the initial reports from the PARTNER trial (9,10). A meta-analysis evaluating the trend in the rates of neurological complications for patients undergoing TAVR found a significant decline in rates of stroke attributable to improved valve designs and operator experiences (11).
A recent report from the PORTICO IDE trial raised concern about the possibility of TAVR valve leaflet thrombosis after one patient suffered a stroke following TAVR and was noted to have reduced leaflet motion on computed tomography (CT) and another asymptomatic patient was discovered to have a similar finding (12). Close scrutiny including multiple CT images revealed that reduced leaflet motion is more common than initially assumed. Cases of reduced leaflet motion due to thrombosis seem to resolve with anticoagulation, giving rise to the theory that subclinical thrombosis is the underlying mechanism for the reduced leaflet motion. Antithrombotic therapy after surgically implanted bioprosthesis has been well defined. This, however, was not true for antithrombotic therapy after TAVR. There are wide variations in the antithrombotic therapy used after TAVR. Ongoing trials, such as the ARTE trial, GALILEO trial and CLOE trial will shed more light on this issue and may result in future strategies to prevent subclinical thrombosis in patients after TAVR.
Carnero-Alcázar and colleagues also found higher rates of vascular complications and PVR in patients with TAVR as compared to SAVR. Although these findings were parallel to the findings of most of the previous trials, the studies included in the analysis mostly utilized older generation valves. Over the years, the larger sheaths employed during deployment of the older generation valves have been replaced with the low-profile delivery mechanisms both for the balloon-expandable and self-expandable valves. This was seen in the Sapien 3 (S3) observational study which found comparable rates of vascular complications between TAVR with the low profile S3 valve and SAVR (9,10). The S3 valve also carries the advantage of the polytetrafluoroethylene (PTFE) skirt which has been shown to reduce the rates of PVR, a feature not present in the earlier generation Sapien valves (9,10). Because of very favorable results of the S3 valve in the intermediate risk cohort, the Food and Drug Administration (FDA) recently approved the S3 valve for use in intermediate-surgical-risk patients.
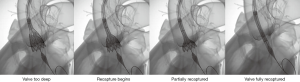
The Evolut R valve, the newer generation self-expandable valve, carries the benefit of a low profile delivery, which will likely transfer into a lower risk of vascular complications as well. In addition, the repositionable and retrievable nature of the Evolut R valve gives operators an additional level of confidence while deploying the percutaneous valves (Figure 1). The early experiences with Evolut R in the US have shown encouraging results (13). The results of the SURTAVI trial, which is studying this valve in intermediate risk patients, are expected soon.
Carnero-Alcázar et al. also found significantly higher rates of pacemaker implantations with the TAVR valve. A study by Siontis et al. found the complication to be 2.5 times more common with the self-expandable valve as compared to balloon expandable valve, a finding reciprocated by a meta-analysis as well (14,15). Deeper implantation into the left ventricular outflow tract and the continual outward radial force applied by the valves are possible reasons for these higher rates with the self-expandable valve as compared to balloon expandable valve (14). The rates of permanent pacemaker implantation and conduction abnormalities were found to be higher with the new S3 system, as compared to older generation balloon expandable valves. This is felt to be due to prosthesis oversizing, the presence of the outer skirt, and the depth of the implantation into the left ventricular outflow tract (16). The higher rates of pacemaker implantation with the newer generation valves are a cause of concern and will have to be addressed.
Recognizing the need for minimally invasive approaches after the advent of TAVR, SuAVR has recently emerged as a feasible alternative in high-surgical-risk patients. Sutureless prostheses reduce the need for sutures after annular decalcification and reduce aortic cross-clamp and cardiopulmonary bypass (CPB) duration (17). In cardiac surgery, prolonged CPB and cross-clamp durations are strong independent risk factors for postoperative mortality and morbidity (18,19). These detrimental effects are further augmented in patients with multiple co-morbidities. This can potentially explain the lower rates of mortality with SuAVR as compared to TAVR in the analysis by Carnero-Alcázar et al. As compared to TAVR, SuAVR was found to have comparable rates of bleeding complications. Furthermore, the benefit of TAVR in terms of lower rates of acute renal failure was attenuated when compared to SuAVR alone. The reduced rates of bleeding with SuAVR were attributed to limited wound bleeding due to the minimally invasive technique used, a mechanism similar to TAVR (20). Lower rates of bleeding have been associated with reduced rates of acute renal failure, which can explain the lower rates of acute renal failure with SuAVR as well (21,22). However, SuAVR suffered from the higher rates of pacemaker implantation rates as seen with TAVR. The mechanism is again similar to TAVR as SuAVR is performed by the deployment of balloon-expandable or self-expandable stents. Despite some success in observational studies, the practical application of SuAVR remains limited due to the lack of randomized trials and data on durability.
Currently, SAVR continues to be the only modality approved for patients with low surgical risk of surgery. There is a scarcity of data for TAVR in lower-surgical-risk patients with only a small meta-analysis showing reasonable outcomes for TAVR in short-term (23). The valves used for TAVR and SuAVR continue to lack data on durability which is crucial in younger, lower risk patients with longer life expectancy. A 2016 report from Dvir et al. raised concerns about the TAVR valve, showing the degeneration rates at almost 50% in 8 years (24). Issues have been raised regarding the mechanical effect of balloon-expansion on valve degeneration (25). However, other reports such as the report by Douglas et al. from the PARTNER ECHO registry have calmed down some of these doubts by showing reasonable levels of degeneration and showing re-intervention and mortality to be associated with pre-TAVR low ejection fraction and low stroke volumes rather than valve degeneration (26). Italian TAVR investigators were amongst the first to use the self-expandable CoreValve and did not show significant valve deterioration at the 5-year period (27). Another challenge for expanding TAVR in to the lower risk cohort will be the high prevalence of bicuspid aortic valves in the younger age group. The extreme and asymmetrical calcification often seen with bicuspid valves can prevent adequate expansion of the valve frame and affect valve hemodynamics leading to higher aortic valve gradients and higher paravalvular leaks (PVL); whereas the higher incidence of aortopathy associated with bicuspid AS can lead to higher rates of aortic dissection (28). Although new data have emerged showing the feasibility in performing TAVR in bicuspid valves with newer generation TAVR valves (29), there will be little tolerance for adverse outcomes for the low-risk category patients as they will continue to be excellent candidates for surgery. In the meantime, clinical trials involving both the Medtronic CoreValve Evolut R system (NCT02701283) and the Edwards S3 system (PARTNER 3 trial, NCT02675114) in low-risk patients are currently ongoing.
The evolution of valve prosthesis, lower profile delivery systems, and increasing operator experiences have extended the reach of minimally invasive valve replacement via transcatheter methods to intermediate-surgical-risk patients in addition to inoperable and high risk patients with very favorable results. The goal to extend TAVR to low risk patients will largely depend on results of durability and the feasibility in bicuspid aortic valves (30). This meta-analysis helps guide our expansion of this technology while we await the results of the randomized clinical trials.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002;106:3006-8. [Crossref] [PubMed]
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [Crossref] [PubMed]
- Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790-8. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2438-88. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609-20. [Crossref] [PubMed]
- Arora S, Misenheimer JA, Jones W, et al. Transcatheter versus surgical aortic valve replacement in intermediate risk patients: a meta-analysis. Cardiovasc Diagn Ther 2016;6:241-9. [Crossref] [PubMed]
- Carnero-Alcázar M, Maroto LC, Cobiella-Carnicer J, et al. Transcatheter versus surgical aortic valve replacement in moderate and high-risk patients: a meta-analysis. Eur J Cardiothorac Surg 2017;51:644-52. [PubMed]
- Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016;387:2218-25. [Crossref] [PubMed]
- Herrmann HC, Thourani VH, Kodali SK, et al. One-Year Clinical Outcomes With SAPIEN 3 Transcatheter Aortic Valve Replacement in High-Risk and Inoperable Patients With Severe Aortic Stenosis. Circulation 2016;134:130-40. [Crossref] [PubMed]
- Athappan G, Gajulapalli RD, Sengodan P, et al. Influence of transcatheter aortic valve replacement strategy and valve design on stroke after transcatheter aortic valve replacement: a meta-analysis and systematic review of literature. J Am Coll Cardiol 2014;63:2101-10. [Crossref] [PubMed]
- Makkar RR, Fontana G, Jilaihawi H, et al. Possible Subclinical Leaflet Thrombosis in Bioprosthetic Aortic Valves. N Engl J Med 2015;373:2015-24. [Crossref] [PubMed]
- Popma J, Dibu G, Hughes GC, et al. Transcatheter Aortic Valve Replacement with a Repositionable Self-expanding Bioprosthesis in Patients With Severe Aortic Stenosis at High Risk for Surgery: One-Year Results from the Evolut R US Pivotal Study. Journal of the American College of Cardiology 2016;68:B16. [Crossref]
- Siontis GC, Jüni P, Pilgrim T, et al. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta-analysis. J Am Coll Cardiol 2014;64:129-40. [Crossref] [PubMed]
- Agarwal S, Parashar A, Kumbhani DJ, et al. Comparative meta-analysis of balloon-expandable and self-expandable valves for transcatheter aortic valve replacement. Int J Cardiol 2015;197:87-97. [Crossref] [PubMed]
- Husser O, Pellegrini C, Kessler T, et al. Predictors of Permanent Pacemaker Implantations and New-Onset Conduction Abnormalities With the SAPIEN 3 Balloon-Expandable Transcatheter Heart Valve. JACC Cardiovasc Interv 2016;9:244-54. [Crossref] [PubMed]
- Gilmanov D, Miceli A, Bevilacqua S, et al. Sutureless implantation of the perceval s aortic valve prosthesis through right anterior minithoracotomy. Ann Thorac Surg 2013;96:2101-8. [Crossref] [PubMed]
- Salis S, Mazzanti VV, Merli G, et al. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J Cardiothorac Vasc Anesth 2008;22:814-22. [Crossref] [PubMed]
- Al-Sarraf N, Thalib L, Hughes A, et al. Cross-clamp time is an independent predictor of mortality and morbidity in low- and high-risk cardiac patients. Int J Surg 2011;9:104-9. [Crossref] [PubMed]
- Phan K, Tsai YC, Niranjan N, et al. Sutureless aortic valve replacement: a systematic review and meta-analysis. Ann Cardiothorac Surg 2015;4:100-11. [PubMed]
- Bagur R, Webb JG, Nietlispach F, et al. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J 2010;31:865-74. [Crossref] [PubMed]
- Elhmidi Y, Bleiziffer S, Piazza N, et al. Incidence and predictors of acute kidney injury in patients undergoing transcatheter aortic valve implantation. Am Heart J 2011;161:735-9. [Crossref] [PubMed]
- Arora S, Strassle PD, Ramm CJ, et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Lower Surgical Risk Scores: A Systematic Review and Meta-Analysis of Early Outcomes. Heart Lung Circ 2017. [Crossref] [PubMed]
- Dvir D. First look at long-term durability of transcatheter heart valves: assessment of valve function up to 10 years after implantation. Presented at: EuroPCR 2016 May 17. Paris, France.
- Arora S, Ramm CJ, Misenheimer JA, et al. Early transcatheter valve prosthesis degeneration and future ramifications. Cardiovasc Diagn Ther 2017;7:1-3. [Crossref] [PubMed]
- Douglas PS. Mid-term hemodynamic trends and between echo changes in transcatheter aortic valves in the PARTNER 1 trial: 5-year results. Presented at: TCT 2016 November 1. Washington DC, USA.
- Barbanti M, Petronio AS, Ettori F, et al. 5-Year Outcomes After Transcatheter Aortic Valve Implantation With CoreValve Prosthesis. JACC Cardiovasc Interv 2015;8:1084-91. [Crossref] [PubMed]
- Makkar R, Chakravarty T, Jilaihawi H. Transcatheter Aortic Valve Replacement for Bicuspid Aortic Stenosis: Are We Ready for the Challenge? J Am Coll Cardiol 2016;68:1206-8. [Crossref] [PubMed]
- Yoon SH, Lefèvre T, Ahn JM, et al. Transcatheter Aortic Valve Replacement With Early- and New-Generation Devices in Bicuspid Aortic Valve Stenosis. J Am Coll Cardiol 2016;68:1195-205. [Crossref] [PubMed]
- Arora S, Ramm CJ, Strassle PD, et al. Review of Major Registries and Clinical Trials of Late Outcomes After Transcatheter Aortic Valve Replacement. Am J Cardiol 2017. [Epub ahead of print]. [Crossref] [PubMed]