Feasibility of four-arm robotic lobectomy as solo surgery in patients with clinical stage I lung cancer
Introduction
Lung cancer screening programs with low-dose chest computed tomography in at-risk populations are resulting in increased numbers of patients with early-stage non-small cell lung cancer (NSCLC) that is potentially best removed by minimally invasive surgical approaches (1). As a form of minimally invasive surgery, video-assisted thoracic surgery (VATS) has been recommended in patients with early-stage NSCLC (2). Video-assisted thoracoscopic lobectomy (VATL) showed acceptable and better postoperative outcomes in terms of postoperative pain, cosmetics, preservation of pulmonary function, and immunosuppression (3,4), and recent studies also showed that VATL showed equivalent oncologic outcomes to conventional open lobectomy (5). However, VATL has several limitations such as its two-dimensional view and restrictive instrumental movement (6).
Robotic surgery has advantages over VATS, including a steady camera with three-dimensional vision, wristed instruments, and improved ergonomics for the surgeon (6). Based on these advantages, robotic surgery has been introduced in certain fields of thoracic surgery such as mediastinal tumor excision, esophageal cancer, and lung cancer (7-10). As robotics can provide three-dimensional visualization and greater instrument maneuverability in a confined space, it has the potential of enhancing minimally invasive thoracoscopic lobectomy (11). In a previous study, robotic lobectomy for early-NSCLC was reported to be feasible and safe (8). In addition to the technical benefits of a robotic technique over VATS, the robotic system has another advantage in that conventional VATL requires an experienced endoscopist and assistant, whereas the surgeon can manipulate the scope and instruments alone in robotic surgery. Solo surgery can be defined as a practice in which a surgeon operates alone, without other surgical members except a scrub nurse (12). We hypothesized that a robotic system using the fourth arm of the da Vinci Robotic System could enable solo surgery without the need for an experienced endoscopist or assistant. In the surgical field, and especially in cardiothoracic surgery, the lack of human resources has been a problem, and we thought that solo surgery might be a solution to this obstacle. Therefore, this prospective study was performed to investigate the possibility and feasibility of the four-arm robotic lobectomy (FARL) technique as solo surgery in patients with early-stage NSCLC.
Methods
Patients and study design
We performed a prospective, patient-preference accrual study (IRB No. 1-2010-0054) that was planned in patients with clinical stage I NSCLC. The exclusion criteria were patients aged <20 years or >80 years, presence of hilar lesions, contraindication for one-lung ventilation, or severe pleural adhesion. The study period was March 2011 to February 2013, and the patients chose between FARL and VATL as their preferred surgical technique. The study was designed with two stages. Interim analysis of early postoperative outcome was performed after the initial 10 cases in each group to test the feasibility and safety of the FARL technique. If the life-threatening complication rate was >10% or the thoracotomy conversion rate was >20%, we planned early termination of the study. Otherwise, we would continue the study with 10 additional cases per group. The 5-year overall survival and disease-free survival rates were calculated in both groups after completion of the study.
We collected data on the patients’ basic clinical characteristics (age, sex, preoperative pulmonary function, smoking history), operative outcomes [operation time, anesthesia time, blood loss, and number of dissected lymph nodes (LNs)], postoperative outcomes (complication, duration of chest tube drainage, and length of hospital stay), pathological data, and long-term oncologic outcomes. All procedures were performed by a surgeon who had already performed more than 200 VATL procedures prior to beginning the robotic lobectomy and who also had abundant experience with other robotic thoracic surgeries (13).
Definitions
As an operative outcome, the lobectomy time was defined as time from the initiation of lobectomy to the retrieval of lung specimen. The anesthesia time was defined as time from the initiation of anesthesia to the finish of anesthesia after extubation. Life-threatening event was defined as any event that developed during the operation and was potentially fatal, such as injury of major vessel or bronchus. The operation time was defined as time from the skin incision to the closure of skin wound. Pain was assessed by nurses using a numeric pain visual analogue scale (VAS) every 8 hours and after interventions for pain control with intravenous analgesia. Prolonged air leakage was defined as leakage lasting beyond the 7th postoperative day (PODs) (14). Postoperative complications were recorded based on the Common Terminology Criteria for Adverse Events (CTC-AE) version 4.0 (15). Local recurrences were defined as those occurring on resection margins, such as bronchial stumps or stapler lines. Regional recurrences were defined as those occurring in the hilar or mediastinal LNs, pleural cavity, and ipsilateral lung. Distant recurrences were defined as those occurring in the contralateral lung, brain, liver, adrenal glands, bone, or other locations.
Surgical technique
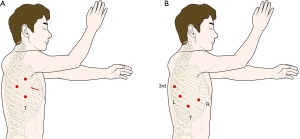
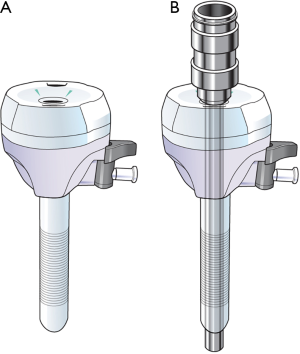
All procedures were performed in the lateral decubitus position. VATL was performed using one working window and three thoracoscopic port incisions: 12 mm thoracoscopic port at 6th intercostal space (ICS) midaxillary line, 12 mm port at the 5th ICS scapular tip, 5-mm port at the 3rd ICS midaxillary line, and 4 cm working window at the 5th ICS anterior axillary line (Figure 1A). One surgeon, one assistant, and one endoscopist participated in VATL. Robotic lobectomy was performed using the da Vinci Si system (Intuitive Surgical, Inc., Mountain View, CA) with the four-arm complete portal approach: 12 mm thoracoscopic port at the 8th ICS midaxillary line, 12 mm port at the 7th ICS posterior axillary line for the left arm, 8 mm port at the 6th ICS scapular tip for the third arm, and 8 mm port at the 6th ICS anterior axillary line for the right arm (Figure 1B). FARL was performed as a fully endoscopic procedure with CO2 inflation without a working window. For specimen retrieval, a small skin incision was made at the end of the operation. We used the double cannulation technique for the left arm, which uses an 8-mm port inserted in a 12 mm accessory port for maintain the CO2 inflation during the operation (Figure 2A). For the insertion of robotic instruments, 8 mm trocar was inserted through the 12 mm port. For the retrieval of dissected LNs and insertion of gauze, 12 mm port was used after removing the 8 mm trocar (Figure 2B). The 5 mm thoracic grasper, 8 mm curved bipolar dissector and fenestrated bipolar forceps were used for robotic procedure. One surgeon and one bed-side assistant participated in FARL. All procedures such as dissection, retraction of lung, and vessel isolation were performed by the surgeon, and the assistant played a minimal role, such as retrieval of dissected LNs and insertion of gauze. At the end of operation, we have expanded existing ports which located in anterior axillary line about 4 cm to be larger to retrieve the specimen if FARL group.
Statistical analysis
Patient general characteristics were described as mean ± standard deviation (SD) for continuous variables and as number of cases with frequency (%) for categorical variables. The two groups were compared using the Chi-square test or Fisher’s exact test for discrete variables and independent sample t-test for continuous variables. The Kaplan-Meier method and log-rank test were used to calculate and compare the survival rates. All P values were 2-sided, and P value <0.05 was considered significant. Statistical analysis was performed using the open-source statistical software R (http://www.R-project.org).
Results
Basic clinical characteristics
The study was terminated in September 2012 because the interim analysis demonstrated safety issues in the FARL group. Three cases (25%) of life-threatening events were observed in the FARL group; as a result, the study was terminated early after enrollment of 17 patients for the VATL group and 12 patients for the FARL group. The clinical characteristics of the study group are presented in Table 1. No significant differences were found between the two groups for sex, age, smoking history, preoperative pulmonary function, or clinical stage.
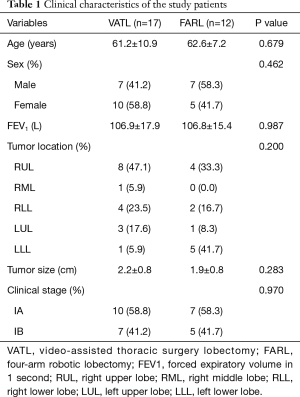
Full table
Operative outcomes and pathologic results
The operative outcomes are summarized in Table 2. Lobectomy time and total operation time (including LN dissection time) were significantly longer in the FARL group (VATL vs. FARL: 47.9±16.0 vs. 81.1±31.4 min for lobectomy time, P=0.009; 134.7±36.5 vs. 195.6±54.2 min for total operation time, P=0.003), whereas there was no significant difference in blood loss between the two groups (132.9±102.5 vs. 151.6±193.2 mL, P=0.738). Three life-threatening events (25.0%) occurred in the FARL group (1 bleeding from pulmonary artery, 1 bleeding from superior branches of pulmonary vein, and 1 bronchial tear), and one patient experienced emergent thoracotomy conversion in order to control bleeding from the superior pulmonary vein. The console surgeon performed anterior-lateral thoracotomy by connecting the previously existed ports because the conventional posterolateral thoracotomy requires more time. In the patient who had pulmonary artery bleeding during right lower lobectomy, bleeding was controlled with FloSeal (Baxter International Inc., Vienna, Austria), and the total bleeding was 150 mL. Another patient had a right middle lobar bronchial tear during the right lower lobectomy and underwent repair of the RML bronchus with polydioxanone suture. The incidence of adverse events was not different between the two groups (P=0.297).
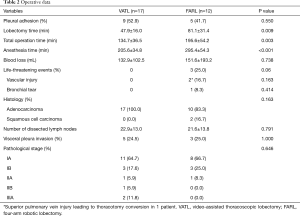
Full table
Pathological staging revealed that 14 patients in the VATL group (82.3%) had stage I, 2 (11.8%) had stage II, and 2 (11.8%) had stage IIIA cancer. In the FARL group, 11 patients (86.7%) had stage I cancer and 1 (8.3%) had stage II cancer (P=0.646). There were no significant differences in histology and number of dissected LNs (22.9±13.0 vs. 21.6±13.8, P=0.791).
Early postoperative outcome and pathological data
The early postoperative outcomes are summarized in Table 3. No significant differences in highest VAS score at each POD were observed between the groups on PODs 0, 1, 2, and 3 (VATL vs. FARL: 5.76±2.07 vs. 5.83±1.52, P=0.919; 5.47±1.90 vs. 5.42±2.42, P=0.949; 4.12±1.57 vs. 4.25±1.86, P=0.843; 4.41±2.42 vs. 3.75±1.71, P=0.397, respectively). No significant differences were observed in duration of chest tube drainage (3.9±1.9 vs. 5.2±4.1 days, P=0.326) and length of hospital stay (5.5±1.8 vs. 6.9±3.9 days, P=0.268). Postoperative complications occurred in 2 (11.8%) patients in the VATL group and 2 (16.7%) patients in the FARL group (P=1.0). All complications were prolonged air leakage.

Full table
Recurrence and survival analysis
The mean follow-up time was 48.9±9.5 months. Recurrence was detected in 2 (16.7%) patients after FARL and in 3 (23.5%) patients after VATL (P=0.671). In the VATL group, 2 (16.7%) patients had locoregional recurrence: one in the ipsilateral lung and one in the pleura. Three (23.5%) patients in the FARL group suffered from recurrences: one in the ipsilateral lung and two pleural masses. The 5-year overall survival was 100% in the FARL group and 87.5% in the VATL group (log-rank test, P=0.386, Figure 3A), and 5-year disease-free survival was 82.5% in the FARL group and 75.6% in the VATL group (log-rank test, P=0.589, Figure 3B). The overall and disease-free survival rates were comparable between the two groups.
Discussion
VATL has been recommended for early-stage NSCLC (2) because it showed acceptable or better postoperative outcomes as well as equivalent long-term survival in comparisons with conventional open lobectomy (3-5). Recently, robotic lobectomy has been introduced because the robotic system can offer advantages such as three-dimensional vision, wristed instruments, and improved ergonomics for the surgeon (16). Many studies have reported the feasibility and safety of robotic-assisted thoracoscopic lobectomy. Jang et al. showed that the outcome of robot-assisted surgery was significantly more favorable than that of VATL in terms of postoperative complications, intraoperative blood loss, and postoperative length of hospital stay (8). Louie et al. reported that the robotic lobectomy group used fewer analgesics and returned to daily activities earlier than the VATS group, although no significant difference in perioperative outcomes was found between the two groups (9).
The VATS procedure requires an experienced endoscopist and assistant. The visual information is collected by a camera assistant, who controls the endoscope based on the surgeon’s instructions and using a set of empirical rules. This can lead to communication problems between the surgeon and the assistant and to an unsteady camera picture when the assistant has to stand still for a long time (12). The previous settings for conventional robotic lobectomy usually require an experienced bedside surgeon for retraction of the lung, suctioning of retained blood, and stapling. For example, in Louie’s report, the first 30 pulmonary resections used 2 attending surgeons (one on the console and one at the bedside); after 30 cases, a thoracic surgery fellow was used as the bedside assistant (9). The need for two surgeons in one robotic operation is excessive use of medical personnel. We proposed that the surgeon at the console could manipulate both the instruments and the camera. Therefore, we planned complete robotic lobectomy as a solo surgery, without an experienced endoscopist or assistant. Solo surgery has been defined as a practice in which a surgeon operates alone, without other surgical members except a scrub nurse (12). We thought that solo surgery might provide a solution to the worldwide lack of human resources in the surgical field, especially in cardiothoracic surgery. Our main reason for using the robotic system in lobectomy was to minimize the utilization of medical personnel. Therefore, we performed this study to investigate the feasibility and efficacy of FARL as a solo surgery in NSCLC.
In our study, we compared operative, postoperative, and survival data between FARL and VATL. Even though FARL and VATL showed similar operative outcomes and long-term oncologic outcomes, FARL required longer operation time and anesthesia time. Robotic surgery is useful because a high-resolution three-dimensional monitor and flexible instruments provide a wrist-like motion that enables meticulous dissection in a narrow space, such as recurrent laryngeal nerve nods in esophageal cancer (7). During the procedure, we realized that the benefits of robotic surgery were not as prominent as expected because lobectomy with mediastinal LN dissection is a multi-quadrant surgery. In addition, the incidences of life-threatening events were more frequent in FARL group, although with marginal significance (P=0.06). A possible explanation for this is the difficulty in dissecting the posterior part of a target tissue such as vessels of bronchi because of the lack of tactile feedback. In addition, adequate exposure of the operation field was sometimes difficult because retraction of the lung with the fourth arm was cumbersome. Also, the current robotic system does not allow simultaneous action of both the third arm and the fourth arm. Those issues seemed to contribute to the prolonged operation time in FARL, which was significantly longer than that of VATL in our study; the FARL procedure was typically 1 hour longer than VATL. Although we introduced FARL to reduce the use of medical personnel, the total use of resources might be increased because of the prolonged operation time. Another drawback of FARL is that it is very expensive. In the national health insurance system of our county, the patient usually pays 4,000 US dollar after VATL including hospitalization, compared with 12,000 US dollars after FARL. Considering the high cost of robotic surgery and the outcome data, we thought that the transition from VATL to FARL by a surgeon who is already experienced in VATL could not be recommended because of safety issues and economic reasons. The similar results were reported in recent article; Migliore mentioned that the adoption of the robotic system in pulmonary resection is problematic due to no survival benefits, longer duration of the operation and the longer operative room usage and higher cost compared to open or VATS lobectomy (17).
This study has several limitations. First, this study enrolled too few patients because the study was terminated early due to safety issues. The second involves the issue of a learning curve for FARL; however, our surgeon had already performed more than 200 cases of robotic surgery, including esophagectomy and mediastinal tumor excision (13).
In conclusion, this study showed that FARL as a solo surgery failed to demonstrate any benefits over VATL. Considering the safety issues and high cost of robotic surgery, the transition from VATL to FARL may not be recommended for experienced surgeons.
Acknowledgements
Funding: This study was supported by a faculty research grant of Yonsei University College of Medicine for 6-2011-0125.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of Severance Hospital Yonsei University Health System (No. 1-2010-0054).
References
- Veronesi G, Bellomi M, Mulshine JL, et al. Lung cancer screening with low-dose computed tomography: a non-invasive diagnostic protocol for baseline lung nodules. Lung Cancer 2008;61:340-9. [Crossref] [PubMed]
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive Summary: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:7S-37S.
- Demmy TL, Curtis JJ. Minimally invasive lobectomy directed toward frail and high-risk patients: a case-control study. Ann Thorac Surg 1999;68:194-200. [Crossref] [PubMed]
- Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362-5. [Crossref] [PubMed]
- Rueth NM, Andrade RS. Is VATS lobectomy better: perioperatively, biologically and oncologically? Ann Thorac Surg 2010;89:S2107-11. [Crossref] [PubMed]
- Ambrogi MC, Fanucchi O, Melfi F, et al. Robotic surgery for lung cancer. Korean J Thorac Cardiovasc Surg 2014;47:201-10. [Crossref] [PubMed]
- Park SY, Kim DJ, Yu WS, et al. Robot-assisted thoracoscopic esophagectomy with extensive mediastinal lymphadenectomy: experience with 114 consecutive patients with intrathoracic esophageal cancer. Dis Esophagus 2016;29:326-32. [Crossref] [PubMed]
- Jang HJ, Lee HS, Park SY, et al. Comparison of the early robot-assisted lobectomy experience to video-assisted thoracic surgery lobectomy for lung cancer: a single-institution case series matching study. Innovations (Phila) 2011;6:305-10. [Crossref] [PubMed]
- Louie BE, Farivar AS, Aye RW, et al. Early experience with robotic lung resection results in similar operative outcomes and morbidity when compared with matched video-assisted thoracoscopic surgery cases. Ann Thorac Surg 2012;93:1598-604; discussion 1604-5. [Crossref] [PubMed]
- Bodner J, Wykypiel H, Greiner A, et al. Early experience with robot-assisted surgery for mediastinal masses. Ann Thorac Surg 2004;78:259-65; discussion 265-6. [Crossref] [PubMed]
- Meyer M, Gharagozloo F, Tempesta B, et al. The learning curve of robotic lobectomy. Int J Med Robot 2012;8:448-52. [Crossref] [PubMed]
- Jaspers JE, Breedveld P, Herder JL, et al. Camera and instrument holders and their clinical value in minimally invasive surgery. Surg Laparosc Endosc Percutan Tech 2004;14:145-52. [Crossref] [PubMed]
- Park SY, Kim DJ, Do YW, et al. The oncologic outcome of esophageal squamous cell carcinoma patients after Robot-Assisted thoracoscopic esophagectomy with total mediastinal lymphadenectomy. Ann Thorac Surg 2017;103:1151-7. [Crossref] [PubMed]
- Cerfolio RJ, Bass CS, Pask AH, et al. Predictors and treatment of persistent air leaks. Ann Thorac Surg 2002;73:1727-30; discussion 1730-1.
- US Department of Health and Human Services, et al. Common terminology criteria for adverse events (CTCAE) version 4.0. National Cancer Institute, 2009, 09-5410.
- Brooks P. Robotic-Assisted thoracic surgery for Early-Stage lung cancer: a review. AORN J 2015;102:40-9. [Crossref] [PubMed]
- Migliore M. Robotic assisted lung resection needs further evidence. J Thorac Dis 2016;8:E1274-8. [Crossref] [PubMed]



