Vascular approaches for transcatheter aortic valve implantation
Introduction
Valvular heart disease is frequently manifested as calcific aortic stenosis (AS), and aortic valve replacement (AVR) is the only effective treatment once symptoms have developed. Despite an overall low mortality rate (1), surgical AVR represents a high-risk procedure in old and frail patients, often carrying due to several coexisting conditions (2). A decade ago, extremely high-risk or non operable patients with aortic valve disease had few options besides palliative care. Cribier et al. (3) performed the first human transcatheter aortic valve implantation (TAVI) in 2002, a procedure typically targeted at patients with severe AS who were unfit for conventional surgery (4-6). In these patients, TAVI offers a solution by relieving the left ventricular outflow track obstruction in a durable and controlled manner, in comparison with balloon valvuloplasty (7). Multiple studies have documented favorable outcomes of this new therapy using a number of endpoints, including survival, symptom status, quality of life, and need for repeat hospitalizations (8). A new paradigm came forth, and TAVI is now endorsed in current guidelines with a class I indication for symptomatic patients with severe AS who are not candidates for surgery and with class IIa indication in AS patients who are at high risk of dying or complications after surgery (9,10). The purpose of this article is to specifically review the different available vascular access approaches when treating an AS patient with TAVI (11).
Vascular screening for TAVI procedure
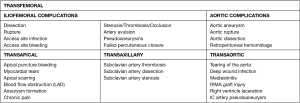
TAVI is technically achievable in almost all AS patients. A wide range of specific anatomic evaluations, however, must be born in mind when referring the appropriate candidates for TAVI procedure. Large sheaths and catheters used in these interventions increase the risk of vascular complications, which have stablished as the main limiting factor of the technique (Figure 1). They can increase early and late mortality, so it is essential to choose an appropriate approach.
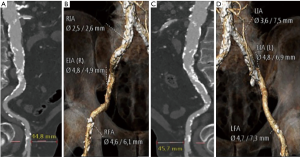
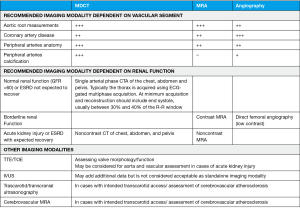
Outlining TAVI approach requires a deep understanding of vascular features such as luminal size, tortuosity and vessel calcification load. This can be achieved with multidetector computed tomography (MDCT) systems provided they have at least 64 detectors and 0.5 to 0.6 mm of spatial resolution (Figure 2) (12-14). Nephrotoxicity risks of iodinated contrast agents (standard protocols use injection of 80–120 mL of low-osmolar agents) must be weighed against procedure benefits, especially with increasing age (14-16). If these contrast agents are absolutely contraindicated, vascular access can be alternatively assessed by magnetic resonance imaging and, valve size, by transesophageal echocardiogram (14) (Figure 3).
Transfemoral
Current consensus and expertise strongly favor the femoral artery as the preferred and most widespread access site for TAVI. Both surgical cut down or percutaneous approaches are feasible to entry the femoral artery. The percutaneous puncture and suture pre-closure techniques are the preferred approaches to entry and seal the femoral artery, and can be performed under loco-regional anesthesia (17). However, up to 20% of cases need an open surgical access, and this percentage might increase as it does the complexity of the patients referred (18,19) (Figure 4). If needed, conversion of percutaneous insertions into open or hybrid repairs is possible using percutaneous closure devices and surgical techniques. Damaging of ilio-femoral vessels is the main threat of the femoral approach. The ideal location for common femoral puncture is between the inferior epigastric artery and the femoral bifurcation. Other requirements for currently available sheaths (14–20 F TAVI delivery catheters) are a minimum femoral and iliac diameter of 6–6.5 mm, with limited vascular calcification and tortuosity. Some situations are deemed preferable for a surgical cut down, such as obese individuals, presence of femoral grafts or stents or any condition requiring a high puncture (i.e., a high femoral bifurcation) (20). When this is not feasible, or in patients not meeting minimal vascular requirements, a non-transfemoral approach should be considered to avoid vascular complications, which entail a worse prognosis (21).
Once the femoral artery has been accessed, the catheter is moved forward to the aortic valve. Valve unfolding is carried out by transcatheter introduction of a balloon or self-expandable valve under high rate ventricular pacing (180–200 bpm) aimed to reduce heart movement (2). Following TAVI deployment, the delivery complex is withdrawn, anticoagulation state is restored and access site is sealed. It is advisable to perform a descending aortic angiogram after removing the sheath and completing percutaneous closure in order to exclude vascular complications (i.e., aortic or iliofemoral perforations/dissections).
With valve delivery catheters decreasing in size, TAVI procedure can now be done completely percutaneously. Nevertheless, peripheral artery disease and AS often share cardiovascular risk factors; it is not surprising that many TAVI candidates are also affected by concomitant, severe peripheral vasculopathy. Because the femoral approach is contraindicated in patients with serious femoral/iliac atherosclerosis, calcifications or tortuosity, and should be regarded guardedly in those with thoracic/abdominal aortic aneurysm, alternative access sites and approaches have been formulated to enable them to undergo transcatheter procedures.
Transapical
The transapical approach was initially described by Ye et al. (22) in 2006 and represents an alternative access route for those cases with non-viable femoral access (23). The procedure requires general anesthesia and is optimally performed in a hybrid surgery room. After a left anterolateral mini-thoracotomy along the fifth or sixth intercostal space the pericardium is opened and left ventricular apex is exposed (Figure 5A). A double purse-string suture with Teflon or pericardium is placed around the puncture site. Direct puncture is followed by direct left ventricular apex sheath insertion. The aortic valve is crossed with a guide wire and the rest of the protocol for valve deployment parallels the one described for transfemoral approach. Once the valve has been deployed, sheath is withdrawn and sutures are tied under rapid ventricular pacing to keep low pressure until repair completion.
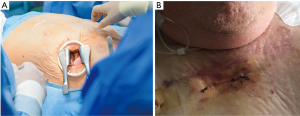
Complications during transapical procedure might be related to: left ventricular pricking (direct myocardial or mitral injury), bleeding, hemodynamic or respiratory compromise and thoracotomy pain. This technique is particularly recommended in cases with high risk of stroke or other embolic events, such as patients with advanced peripheral artery disease or severely calcified thoracic aorta (porcelain aorta) (24,25).
Transaxillary/subclavian
The transaxillary/subclavian access route is safe and represents an increasingly accepted option in TAVI candidates with contraindication to both transfemoral and transapical approaches (26). It was the first alternative access site gaining the Conformité Européene approval in 2010. Approaching the subclavian artery can be performed with local anesthetic and mild sedation, followed by an easy surgical cutdown from the deltopectoral groove to the pectoralis major: dissection or retraction of the pectoralis would then yield exposition of the subclavian artery. This avoids the invasiveness of the transapical technique and overcomes peripheral vascular disease. However, the subclavian artery might get damaged in certain situations (diameter <6 mm; severe calcification/tortuosity or a fixed stenosis not suitable for angioplasty) that should be carefully excluded before the procedure (27). It should also be born in mind the location of the brachial plexus just above the subclavian artery. A multidisciplinary team is needed to balance this vascular features and longer procedural times (when compared to percutaneous transfemoral implantation) with clear advantages of the technique, such as enabling a more rapid mobilization of patients.
Once the artery has been located, it is tied in the anterior wall of the artery with a purse-string suture of 5-0 polypropylene. Then, a 6-Fr sheath is placed over with a soft, J-tip 0.035 wire. This wire would be used to insert a catheter into the ascending aorta, and then switched for a stiff Amplatz wire for introduction of dilators of 10, 12, 14, and 18 Fr. This latter 18-Fr sheath can be then be advanced through the subclavian artery into the proximal ascending aorta. The following steps for device deployment follow the standard protocol of the intervention. After the sheath is removed, the purse-string suture is tied under direct visualization that would determine if additional sutures are needed.
When using the subclavian artery, some considerations should be taken (28):
- Right subclavian artery can be used, but device positioning becomes technically difficult when the plane of the valve (transverse plane) and the horizontal plane form an angle ≥30 degrees;
- If the left subclavian artery has been previously used in a coronary artery graft, left internal mammary artery flow might be impaired. In this setting, sheath insertion and device placement must be carefully done by fluoroscopic guidance that would rule out sheath obstruction or artery injury (with subsequent ischemia);
- Complete sheath introduction in cases of calcified/not too large subclavian arteries or right vertebral artery obstruction (and left vertebral artery dominance) should only be performed at the time of prosthesis delivery into the aortic arch. Then, the sheath would be slightly retrieved so as to reduce the risk of mammary artery dissection/occlusion (28).
Continuous advances of this technique have recently leaded to publications of fully percutaneous procedures without surgical cutdown (29,30). However, these improvements were not able to displace surgical exposure as the routine practice (31-34). Furthermore, despite recognized potential benefit over other techniques, this approach for TAVI has not already gained fully universal acceptance (35).
Direct aortic access
Transaortic access is an alternative TAVI approach achievable through a mini-sternotomy or a right thoracotomy (Figure 5B), both ways allowing exposure of the proximal ascending aorta (Figure 6). Selecting the right access for a given patient is critically important, and this decision mainly relies on technical feasibility and easiness: a right anterior thoracotomy would be preferred if the aorta is to the right side and close to the rib cage; on the contrary, a mini-sternotomy would be easier to perform if the aorta is in the midline or deeper (36,37). The pericardium is exposed and stitched to the skin borders, building a secluded stage that keeps surgical work apart from lung and mediastinal tissues. The operator sutures a purse at the intended access site and places a needle in its center. Then, a 6-Fr sheath is lain over with a soft, J-tip 0.035 wire. This wire would guide the advance through the ascending aorta up to the aortic valve. The close distance to the insertion site and skipping the tension that would have been generated coming around the arch facilitate valve insertion and shorten the operator’s learning curve. After valve deployment, the operator would stitch the purse strings under direct vision similar to decannulation after cardiopulmonary bypass. Closing the chest wall follows standards of common surgery interventions. While the hemisternotomy preserves the pleura intact and usually offers a wider aorta field, the thoracotomy spares patent coronary bypass grafts (generally on the left aortic aspect).
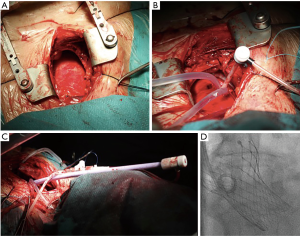
Direct aortic access, although the invasiveness of a mini-thoracotomy and aortotomy, has several advantages. This access route is technically feasible, familiar and easy to learn for cardiac surgeons. In addition, it has been associated with favorable outcomes, and a lower rate of complications (bleeding, risk of myocardial injury) and intensive care unit stay when compared to the transapical approach (38). Advantages over transfemoral or transaxillary approaches include eluding smaller arteries (iliofemoral or the subclavian) by direct insertion of the sheath in the aorta; this cuts down the risk of complications. Moreover, approaching the aortic valve might be a direct upright line, thus facilitating the correct placing of the valve, particularly in a horizontal root.
Transcarotid
Transcarotid approach via the common carotid artery represents a direct route to the aortic valve that shortens the distance from the arterial entry site to the aortic root. As with transapical and transaortic accesses, this improves movement precision and favours sheath and delivery catheter stability. Valve positioning with transcarotid approach is more accurate than via transfermoral.
This approach might be performed under local anesthesia through a small incision in the neck (39), provided that the patient tolerates temporary unilateral carotid occlusion and has adequate anterior communicating artery at the circle of Willis. This can be explored by a transitory shunt into the common carotid; the presence of passive antegrade carotid perfusion would warrant adequate cerebral perfusion during the intervention (40).
Modine and colleagues reported the first case of transcarotid TAVI in an 89-year-old man with symptomatic degenerative AS (41). In a subsequent report by the same group involving 12 consecutive patients who underwent TAVI via the proximal left common carotid artery under general anesthesia, prosthesis implantation was uneventful, and no vascular complications or bleeding were observed. However, one patient had an embolic transient ischemic attack contralateral to the carotid access site (42). More recently, Azmoun and colleagues reported favorable outcomes of 18 out of 19 patients who underwent TAVI (4 Edwards SAPIEN XT and 14 Medtronic CoreValve) by the carotid approach under local anesthesia. No single complication related to vascular access site, myocardial infarctions, strokes or major bleeding were reported. One patient died during preimplant balloon valvuloplasty, and other one death before hospital discharge because of multisystem organ failure. Three patients received a permanent pacemaker due to third-degree atrioventricular block development (39). Future research is warranted to deepen the feasibility of this approach as an alternative for patients who do not meet the criteria for any other access route.
Caval-aortic
The transcaval aortic access is a relatively new technique intended for TAVI in candidates not suitable for other arterial approaches. The abdominal aorta is accessed from the femoral vein through the adjoining inferior vena cava, located close to the abdominal aorta and without interposed structures. Iliofemoral veins are larger and more compliant than corresponding arteries, with a low risk of bleeding (and subsequent hemodynamic compromise) from traumatic or aneurismal fistulas (43). Aortic bleeding is shunted to a patent cava hole because of the pressure applied from the adjacent retroperitoneal space (43). After prosthesis insertion, the caval-aortic tract is closed using a nitinol cardiac occluder device.
The first reported human cohort included elderly patients in whom transfemoral, transapical, or transaortic accesses were not feasible (44). Residual aorto-caval fistulae, hemorrhages requiring transfusion and implantation of covered stents were common. Procedural times with this approach are not longer than those with transfemoral ones, even when considering pre-procedural vascular stitching, cross-over protection and hemostatic techniques (ballon inflation). Moreover, the number of puncture attempts and crossing-closing durations are being increasingly lowered, with a short learning curve that helps operators gain experience and confidence in the procedure.
Preprocedural imaging assessment becames essential for an adequate candidate selection. Contrast-enhanced CT can be used for calcification and vascular diameter, trajectory and tortuosity assessment. This imaging modality helps identify adjacent calcium-free aortic crossing sites without interposed structures or vascular branches. Porcelain aorta is defined by heavily calcification of aortic walls. This condition is characterized by extreme vascular rigidity that hampers aortotomy, catheterization and closure with the nitinol occluder device. Therefore, an alternative approach should be offered to patients with abdominal porcelain aorta. Careful should also be paid if the iliac vessels are severely narrowed, since bailout endovascular graft placement in the abdominal aorta might be difficult to perform.
This technique is subjected to potential pitfalls that restrict this method to experienced operators in properly equipped centers and to patients with certain anatomic features (45). Ideal candidates include patients with proximity between the aorta and the inferior vena cava, a calcium-free window at the intended caval–aortic crossing target, aortic luminal diameter large enough for deployment of a closure device, and absence of important interposed structures (i.e., duodenum, lumbar arteries). The target zone should be at least 1 cm away from the aortic bifurcation, lowest renal artery, or left renal vein to avoid encroachment by the occluder device. Anticoagulation is recommended to avoid aortic or caval thrombosis, although this complication has not yet been described transcaval approach (44). Prospective studies comparing the outcomes of this procedure with the existing surgical approaches may be warranted.
Comparison of different access routes
Transfemoral route is the most commonly access for TAVI and is the reference to compare outcomes with other different pathways. Transfemoral access had shown significant lower rate of 30 day mortality [OR 0.56 (0.5–0.64)] and lower mortality rate at 1 year [OR 0.68 (0.60–0.75)] in comparison with non-transfemoral access. When individual routes are compared, transfemoral has been associated with significant better results in 30 days mortality rate [OR: 0.57 (0.49–0.66)] compared to transapical access (46). These results were ratified in a specific metanalysis that compared both routes. Transfemoral showed significant lower rate of 30 day mortality [OR 0.59 (0.45–0.76)] and lower but non significant one year mortality rate [OR 0.64 (0.34–1.20)] than transapical pathway (47).
Transaxilar route is feasible and is an alternative pathway when transfemoral access is not suitable. There were no significant differences in 30 days mortality rate between transfemoral versus transaxilar routes [OR 0.88 (0.62–1.25)] but with higher rates of general anesthesia. Higher but non-significant rate of vascular complications was reported with transaxilar access (26,46). In a recently reported cohort including 100 consecutive patients treated with percutaneous transaxillary access for TAVI, mortality rates at 30 days and one year were 6% and 14.8%, respectively (48). Comparison between transaxilar and transapical showed a higher but non-significant 30-day mortality rate [OR 1.56 (1.04–2.36)] (46).
The results of a descriptive study that compared transfemoral versus transaortic versus transapical TAVI have been recently reported. It showed higher but non-significant 30-day and one-year mortality rates in the transapical group, and the authors concluded that transaortic could be an alternative option for cases not suitable for transfemoral access (49). Transaortic access has recently showed good results with a 30-day mortality rate of 6.1% in the largest published cohort (50).
Transcarotid access is a novel feasible access with good initial results, but yet not routinely used (51).
Conclusions
TAVI is a rapidly evolving therapeutic modality currently available for patients with severe AS who are unsuitable for surgery because of technical/anatomical issues or too high-estimated surgical risk. However, the peculiarities of each of the vascular approaches designed for TAVI delivery make it necessary to carefully assess patient’s atherosclerotic load and location, arterial size and tortuosity, and presence of mural thrombus. Transfemoral approach is the preferred TAVI delivery route when possible. Alternative non-transfemoral access options include transaortic, trans-subclavian, and transapical access. Other approaches are also feasible (transcarotid, transcaval, and antegrade aortic) but are restricted to operators and hospitals with specialized skillsets and experience. Several clinical trials are currently ongoing and in the near future the indications for these approaches will likely be better defined and extended to a broader spectrum of TAVI candidates.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Edwards FH, Peterson ED, Coombs LP, et al. Prediction of operative mortality after valve replacement surgery. J Am Coll Cardiol 2001;37:885-92. [Crossref] [PubMed]
- Iribarne A, Easterwood R, Chan EY, et al. The golden age of minimally invasive cardiothoracic surgery: current and future perspectives. Future Cardiol 2011;7:333-46. [Crossref] [PubMed]
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002;106:3006-8. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [Crossref] [PubMed]
- Adams DH, Popma JJ, Reardon MJ. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;371:967-8. [Crossref] [PubMed]
- Popma JJ, Adams DH, Reardon MJ, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol 2014;63:1972-981. [Crossref] [PubMed]
- Zajarias A, Cribier AG. Outcomes and safety of percutaneous aortic valve replacement. J Am Coll Cardiol 2009;53:1829-36. [Crossref] [PubMed]
- Avanzas P, Pascual I, Muñoz-García A, et al. Long-term Follow-up of Patients With Severe Aortic Stenosis Treated With a Self-expanding Prosthesis. Revista Española de Cardiologia. Rev Esp Cardiol (Engl Ed) 2017;70:247-53. [Crossref] [PubMed]
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012;42:S1-44. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:e521-e643. [Crossref] [PubMed]
- Holmes DR Jr, Mack MJ. Transcatheter valve therapy: a professional society overview from the American College of Cardiology Foundation and the Society of Thoracic Surgeons. Ann Thorac Surg 2011;92:380-9. [Crossref] [PubMed]
- Leipsic J, Gurvitch R, Labounty TM, et al. Multidetector computed tomography in transcatheter aortic valve implantation. JACC Cardiovasc Imaging 2011;4:416-29. [Crossref] [PubMed]
- Willson AB, Webb JG, Labounty TM, et al. 3-dimensional aortic annular assessment by multidetector computed tomography predicts moderate or severe paravalvular regurgitation after transcatheter aortic valve replacement: a multicenter retrospective analysis. J Am Coll Cardiol 2012;59:1287-94. [Crossref] [PubMed]
- Jabbour A, Ismail TF, Moat N, et al. Multimodality imaging in transcatheter aortic valve implantation and post-procedural aortic regurgitation: comparison among cardiovascular magnetic resonance, cardiac computed tomography, and echocardiography. J Am Coll Cardiol 2011;58:2165-73. [Crossref] [PubMed]
- Zamorano JL, Goncalves A, Lang R. Imaging to select and guide transcatheter aortic valve implantation. Eur Heart J 2014;35:1578-87. [Crossref] [PubMed]
- Bax JJ, Delgado V, Bapat V, et al. Open issues in transcatheter aortic valve implantation. Part 1: patient selection and treatment strategy for transcatheter aortic valve implantation. Eur Heart J 2014;35:2627-38. [Crossref] [PubMed]
- Guinot PG, Depoix JP, Etchegoyen L, et al. Anesthesia and perioperative management of patients undergoing transcatheter aortic valve implantation: analysis of 90 consecutive patients with focus on perioperative complications. J Cardiothorac Vasc Anesth 2010;24:752-61. [Crossref] [PubMed]
- Ludman PF, Moat N, de Belder MA, et al. Transcatheter aortic valve implantation in the United Kingdom: temporal trends, predictors of outcome, and 6-year follow-up: a report from the UK Transcatheter Aortic Valve Implantation (TAVI) Registry, 2007 to 2012. Circulation 2015;131:1181-90. [Crossref] [PubMed]
- Gilard M, Eltchaninoff H, Iung B, et al. Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med 2012;366:1705-15. [Crossref] [PubMed]
- Toggweiler S, Webb JG. Challenges in transcatheter aortic valve implantation. Swiss Med Wkly 2012;142:w13735. [PubMed]
- Thomas M, Schymik G, Walther T, et al. One-year outcomes of cohort 1 in the Edwards SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) registry: the European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 2011;124:425-33. [Crossref] [PubMed]
- Ye J, Cheung A, Lichtenstein SV, et al. Transapical aortic valve implantation in humans. J Thorac Cardiovasc Surg 2006;131:1194-6. [Crossref] [PubMed]
- Al-Attar N, Himbert D, Descoutures F, et al. Transcatheter aortic valve implantation: selection strategy is crucial for outcome. Ann Thorac Surg 2009;87:1757-62. [Crossref] [PubMed]
- Lichtenstein SV, Cheung A, Ye J, et al. Transapical transcatheter aortic valve implantation in humans: initial clinical experience. Circulation 2006;114:591-6. [Crossref] [PubMed]
- Walther T, Mollmann H, van Linden A, et al. Transcatheter aortic valve implantation transapical: step by step. Semin Thorac Cardiovasc Surg 2011;23:55-61. [Crossref] [PubMed]
- Caceres M, Braud R, Roselli EE. The axillary/subclavian artery access route for transcatheter aortic valve replacement: a systematic review of the literature. Ann Thorac Surg 2012;93:1013-8. [Crossref] [PubMed]
- Petronio AS, De CM, Bedogni F, et al. Safety and efficacy of the subclavian approach for transcatheter aortic valve implantation with the CoreValve revalving system. Circ Cardiovasc Interv 2010;3:359-66. [Crossref] [PubMed]
- Fraccaro C, Napodano M, Tarantini G, et al. Expanding the eligibility for transcatheter aortic valve implantation the trans-subclavian retrograde approach using: the III generation CoreValve revalving system. JACC Cardiovasc Interv 2009;2:828-33. [Crossref] [PubMed]
- van Mieghem NM, Luthen C, Oei F, et al. Completely percutaneous transcatheter aortic valve implantation through transaxillary route: an evolving concept. EuroIntervention 2012;7:1340-2. [Crossref] [PubMed]
- Schafer U, Ho Y, Frerker C, et al. Direct percutaneous access technique for transaxillary transcatheter aortic valve implantation: "the Hamburg Sankt Georg approach JACC Cardiovasc Interv 2012;5:477-86. [Crossref] [PubMed]
- Grube E, Buellesfeld L, Mueller R, et al. Progress and current status of percutaneous aortic valve replacement: results of three device generations of the CoreValve Revalving system. Circ Cardiovasc Interv 2008;1:167-75. [Crossref] [PubMed]
- Tamburino C, Capodanno D, Ramondo A, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation 2011;123:299-308. [Crossref] [PubMed]
- Eltchaninoff H, Prat A, Gilard M, et al. Transcatheter aortic valve implantation: early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur Heart J 2011;32:191-7. [Crossref] [PubMed]
- Cioni M, Taramasso M, Giacomini A, et al. Transaxillary approach: short- and mid-term results in a single-center experience. Innovations (Phila) 2011;6:361-5. [Crossref] [PubMed]
- Laflamme M, Mazine A, Demers P, et al. Transcatheter aortic valve implantation by the left axillary approach: a single-center experience. Ann Thorac Surg 2014;97:1549-54. [Crossref] [PubMed]
- Bapat VN, Bruschi G. Transaortic access is the key to success. EuroIntervention 2013;9 Suppl:S25-32. [Crossref] [PubMed]
- Bruschi G, De MF, Botta L, et al. Direct aortic access for transcatheter self-expanding aortic bioprosthetic valves implantation. Ann Thorac Surg 2012;94:497-503. [Crossref] [PubMed]
- Lardizabal JA, O'Neill BP, Desai HV, et al. The transaortic approach for transcatheter aortic valve replacement: initial clinical experience in the United States. J Am Coll Cardiol 2013;61:2341-5. [Crossref] [PubMed]
- Azmoun A, Amabile N, Ramadan R, et al. Transcatheter aortic valve implantation through carotid artery access under local anaesthesia. Eur J Cardiothorac Surg 2014;46:693-8. [Crossref] [PubMed]
- Guyton RA, Block PC, Thourani VH, et al. Carotid artery access for transcatheter aortic valve replacement. Catheter Cardiovasc Interv 2013;82:E583-E586. [PubMed]
- Modine T, Lemesle G, Azzaoui R, et al. Aortic valve implantation with the CoreValve ReValving System via left carotid artery access: first case report. J Thorac Cardiovasc Surg 2010;140:928-9. [Crossref] [PubMed]
- Modine T, Sudre A, Delhaye C, et al. Transcutaneous aortic valve implantation using the left carotid access: feasibility and early clinical outcomes. Ann Thorac Surg 2012;93:1489-94. [Crossref] [PubMed]
- Halabi M, Ratnayaka K, Faranesh AZ, et al. Aortic access from the vena cava for large caliber transcatheter cardiovascular interventions: pre-clinical validation. J Am Coll Cardiol 2013;61:1745-6. [Crossref] [PubMed]
- Greenbaum AB, O'Neill WW, Paone G, et al. Caval-aortic access to allow transcatheter aortic valve replacement in otherwise ineligible patients: initial human experience. J Am Coll Cardiol 2014;63:2795-804. [Crossref] [PubMed]
- Lederman RJ, Babaliaros VC, Greenbaum AB. How to perform transcaval access and closure for transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2015;86:1242-54. [Crossref] [PubMed]
- Chandrasekhar J, Hibbert B, Ruel M, et al. Transfemoral vs Non-transfemoral Access for Transcatheter Aortic Valve Implantation: A Systematic Review and Meta-analysis. Can J Cardiol 2015;31:1427-38. [Crossref] [PubMed]
- Panchal HB, Ladia V, Amin P, et al. A meta-analysis of mortality and major adverse cardiovascular and cerebrovascular events in patients undergoing transfemoral versus transapical transcatheter aortic valve implantation using edwards valve for severe aortic stenosis. Am J Cardiol 2014;114:1882-90. [Crossref] [PubMed]
- Schäfer U, Deuschl F, Schofer N, et al. Safety and efficacy of the percutaneous transaxillary access for transcatheter aortic valve implantation using various transcatheter heart valves in 100 consecutive patients. Int J Cardiol 2017;232:247-54. [Crossref] [PubMed]
- Arai T, Romano M, Lefevre T, et al. Direct Comparison of Feasibility and Safety of Transfemoral Versus Transaortic Versus Transapical Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 2016;9:2320-5. [Crossref] [PubMed]
- Bapat V, Frank D, Cocchieri R, et al. Transcatheter Aortic Valve Replacement Using Transaortic Access: Experience From the Multicenter, Multinational, Prospective ROUTE Registry. JACC Cardiovasc Interv 2016;9:1815-22. [Crossref] [PubMed]
- Stonier T, Harrison M, Choong AM. A systematic review of transcatheter aortic valve implantation via carotid artery access. Int J Cardiol 2016;219:41-55. [Crossref] [PubMed]


