Atypical histiocytosis in the lung
Introduction
Histiocytic/dendritic cell tumors are rare and may involve multiple organs (1). In the past 50 years, only 10 cases of histiocytic tumors of the lung as the main organ involved have been reported (1-5). Indeed, only 4 cases of atypical histiocytic tumors, in total, have been reported, and have involved the nose, lymph nodes, and skin (1,6,7). This is the first case to report an atypical histiocytic pulmonary tumor, exhibiting infiltrative lesions and multiple air cysts in lungs as the primary findings.
Case report
A 47-year-old Chinese female, presenting with fever and cough of one month duration, was hospitalized on December 31st, 2010. She gave a one month history of fevers, without chills, of up to 38.5 °C, without an obvious source. The fever was mainly present at night, and was accompanied by mild cough and white phlegm production.
She had been diagnosed with SLE (systemic lupus erythematosus, SLE) and lupus nephropathy for ten years and had been receiving prednisone since that time. For the prior 2 years the dose had been 20 mg daily, along with hydroxychloroquine, 400 mg daily. She had no history of hypertension, diabetes, coronary heart diseases, infectious diseases, surgery, trauma, or allergy to drug or food.
Prior to admission, a chest X-ray at another hospital suggested a lung infection. However, it was refractory to penicillin, cefuroxime, and azithromycin. On December 17th, 2010, chest CT showed multiple nodules and bullae in both lungs. She was then referred to our hospital.
Physical examination showed temperature of 36.2 °C, pulse 81/min, respiratory rate 22/min, and blood pressure of 113/75 mmHg. She was conscious and alert. Palpable superficial lymph nodes and thyroid swelling were absent. The trachea was in the midline and thorax and chest wall movement on breathing were symmetrical. On auscultation, rough breath sounds were heard bilaterally, but rales were absent. Cardiovascular, abdominal, and neurological examinations were within normal limits.
CBC (complete blood count, CBC) results: hemoglobin 104 g/L (normal: 110-150 g/L), white blood cell count of 4×109 [normal: (4-10)×109/L]; monocytes 19.8% (normal: 3-8%). Liver function tests: r-glutamyl transferase 193 U/L (normal: 9-40 U/L), alkaline phosphatase 200 U/L (normal: 30-120 U/L), total protein 62 g/L (normal: 64-82 g/L), albumin 36 g/L (normal: 34-50 g/L), globulin 26 g/L (normal: 20-45 g/L). Blood gas analysis (nasal oxygen catheter at 2 L/min): pH 7.46 (normal: 7.35-7.45), carbon dioxide partial pressure 24 mmHg (normal: 35-45 mmHg), oxygen partial pressure 67 mmHg (normal: 80-100 mmHg), oxygen saturation 94.0% (normal: 96-100%). Serum protein electrophoresis: α 13.5% (normal range: 52-68%), β 14.7% (normal: 8.0-13.0%). Anti-SSA autoantibody test was positive, and dsDNA and anti-Sm test both negative.
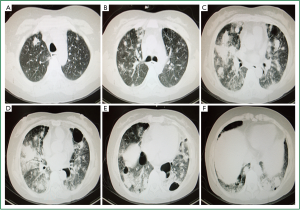
Ultrasound showed a left pleural effusion. Cranial MRI shows minor ischemia and lacunar infarction lesions. Chest CT scan on December 17th, 2010 (Figure 1A-F) exhibited bilateral infiltrative lesions and multiple emphysematous bullae. Endoscopic bronchoscopy revealed patent bronchial lumen with intact mucosa bilaterally. Vegetations were not seen.
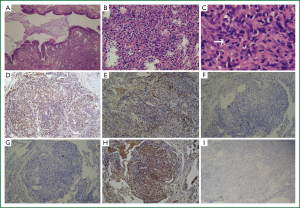
A provisional diagnosis of bilateral lung infection and hypoxemia in a patient with SLE was made. Biapenem, piperacillin-sulbactam, azithromycin, among others, as well as expectorant were given, along with oxygen and other symptomatic supportive treatments. Fevers improved but chest radiographics showed increasing lesions. To make a diagnosis, VATS (video-assisted thoracoscopic, VATS) biopsy was performed. Pathological IHC (immunohistochemistry, IHC) reported on January 26th, 2011 (Figure 2A-I): CD1α (+), CD20 (+), CD3 (+), CD34CD68 (+), Ki-67 (+15%), P53 S-100 (+, but minor), VIM (+), reticulocyte staining (+), D240 (+), CK (board-spectrum) (–), SMA (–), Desmin (–), EMA (–), TTF-1 (–), HMB45 (–), CD207 (–) and CD21 (–), CD35(–), LYS(+). Pathologically, the lesions showed many atypical cells. These were described as mid- or mid-large sized cells in nodular, hyperplastic areas, with prominent nucleoli, loose chromatin, and many abnormal mitoses. Thus, this was considered to be an atypical histiocytic hyperplasia/neoplasm.
Discussion
Histiocytic/dendritic cell tumors are rare. Previously, T-cells or NK-cell lymphoma and some diffuse large B-cell lymphomas were misdiagnosed as histiocytic/dendritic cell tumors (8). Currently, histiocytic/dendritic cell tumors are considered to originate from immune helper cells-macrophages and dendritic cells (1). In addition, advanced techniques in IHC, molecular genetics, and electron microscopy have allowed identification and classification of the histiocytic tumors.
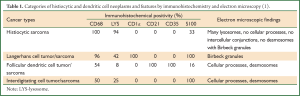
The most widely used classification system is the 2002 classification of the ILSG (International Lymphoma Study Group, ILSG) (1). This immune phenotype-based classification was based on a comprehensive analysis of clinical profiles, morphology, and immune phenotypes of 61 patients (Table 1) (1). This classification system has a diagnostic accuracy rate of up to 93%, with the remaining 7% being diagnosed by morphological and ultrastructural features. The histiocytic/dendritic cell tumor is classified into 4 types: Histiocytic sarcoma, Langerhans cell tumor/sarcoma, Follicular dendritic cell tumor/sarcoma, and Interdigitating cell tumor/sarcoma, with those not so identified being called atypical histiocytic tumor (1).
The final diagnosis for this patient of atypical histiocytic tumor was based on the following evidence: (I) histology showing a nodular distribution, dysplasia of medium-sized cells, and with a proportion of middle-large sized atypical cells. These cells had prominent nucleoli and loose chromatin, with mitoses and abnormal mitoses (Figure 2C, arrow), characteristic of malignancy. This description does not accord with the four types mentioned above, although CD68, S100 and LYS were expressed; (II) IHC: biomarkers for epithelium, including CK, EMA and TIF-1, were negative. However, the monocyte and macrophage-related antigens, CD68 and CD163, were positive; (III) There were no cells with characteristic morphological features of Langerhans cells, such as membrane folds or Birbeck granules. In addition, negative CD1α (Figure 2F), CD21, langerin (Figure 2G), only minor S-100-positivity, and CD20-positivity did not support the diagnosis of LCH (Langerhans cell histiocytosis, LCH). The positive T-cell markers, CD3 and CD20, indicated a lymphocyte-related source instead of a typical histiocytic tumor (in which T-cell and B-cell markers should be negative). Because these findings did not fall into any category of a typical histiocytic tumor (in accord with Table 1), the diagnosis was made of an atypical histiocytic tumor.
Full Table
Clinical manifestations, treatment, and prognosis of histiocytotic tumors
The clinical manifestations of histiocytic/dendritic cell tumors are protean. (I) It is common for each type of histiocytic tumor most frequently seen in lymph nodes, skin, and gastrointestinal tract, with associated hepatosplenomegaly, fever, and weight loss; (II) Multi-organ involvement usually predicts a poorer prognosis; (III) Primary follicular dendritic cell tumor/sarcomas may occur in lymph nodes or extranodal sites such as skin, spleen, and oral mucosa. A few patients show extensive lymph node involvement and splenomegaly. Although relapse after treatment occurs, the majority of patients have a good prognosis; (IV) Interdigitating dendritic cell sarcoma/tumors are rare. These lesions are not confined to lymph nodes, but can involve bone marrow, liver, spleen, skin, lungs and other organs. There are, of course, significant differences in prognoses between local and disseminated disease for all 4 categories.
The primary treatment strategy (7,8) should be to surgically remove the primary tumor, when possible, followed by radiation and chemotherapy. Patients with histiocytic/dendritic cell tumors usually have a poor outcome with a mortality rate of 50% to 60% (8). Treatment options depend on location and involved areas. Chemotherapy, surgery and radiation are potential options.
Our patient initially developed respiratory symptoms without evidence of disease elsewhere. Lesions seemed to be located only in the lungs, exhibiting infiltration and multiple cysts (Figure 1A-F), characteristic of LCH (9).
The patient had a 10-year history of SLE. Although the lung can be involved in SLE, reports are not consistent, but can be as high as 60 percent (10). Pulmonary SLE may involve pleura, lung parenchyma, or pulmonary interstitium, exhibiting dispersed patchy consolidation (11). Characteristics lesions include interstitial fibrosis, vasculitis, hematoxylin bodies, interstitial pneumonia, pleuritis, alveolar wall necrosis, pleural effusion, edema, and alveolar hemorrhage (12). Although there is one case report of a patient with SLE combined with lung histiocytosis (5), SLE combined with atypical histiocytic tumors has not been reported. In the present case, we could not exclude that the lung lesions were from SLE, or that SLE lesions coexisted with atypical histiocytic lesions. Certainly, SLE, with its abnormal immune status, compromised immune surveillance, and potential genetic mutations secondary to chemotherapy might contribute to development of an atypical histiocytic tumor.
Upon discharge treatment was continued with low dose cyclophosphamide, VP16, and vincristine for 6 months through November 2011. With treatment, her symptoms somewhat improved but she continued to have recurrent fevers to 38 °C.
Conclusions
As mentioned above, histiocytic tumors are rare, atypical histiocytic tumors even more so, with pulmonary atypical histiocytic tumors, with infiltrative lesions and multiple cysts, not being previously reported. Our patient’s disease was characterized by non-specific clinical manifestations and imaging findings. Differential diagnosis of similar conditions must be ruled out before reaching such a diagnosis. Pathological evidence is the “gold standard” for a final diagnosis, though immunohistochemistry can aid in its confirmation.
Acknowledgements
Funding: The study was funded by grants from the National Science Foundation of China (No: 81170011); the Science and Technology Commission of Shanghai Municipality (12DJ1400103, 124119a9000, and 12411950105) and the International Science & Technology Cooperation Program of China (2011DFB30010).
Disclosure: The authors declare no conflict of interest.
References
- Pileri SA, Grogan TM, Harris NL, et al. Tumours of histiocytes and accessory dendritic cells: an immunohistochemical approach to classification from the International Lymphoma Study Group based on 61 cases. Histopathology 2002;41:1-29. [PubMed]
- Chang CS, Wang CH, Su IJ, et al. Hematophagic histiocytosis: a clinicopathologic analysis of 23 cases with special reference to the association with peripheral T-cell lymphoma. J Formos Med Assoc 1994;93:421-8. [PubMed]
- Xiao X, Yu B, Yan G, et al. A case report of a primary histiocytotic tumor in lung and literature review. Chinese Journal of Cancer 2008;18:3.
- Guo L, Kang S, Wu D, et al. Two cases of extranodal histiocytotic sarcoma: pathological analysis and literature review. Chinese Journal of Clinical and Experimental Pathology 2007;23:2.
- Li X, Shi H, Wei L, et al. A case report of lung histiocytosis sarcoma. Chinese Journal of Diagnostic Pathology 2007;14:1.
- Flynn KJ, Dehner LP, Gajl-Peczalska KJ, et al. Regressing atypical histiocytosis: a cutaneous proliferation of atypical neoplastic histiocytes with unexpectedly indolent biologic behavior. Cancer 1982;49:959-70. [PubMed]
- de Vicente Rodriguez JC, Santos Oller JM, Junquera Gutierrez LM, et al. Atypical histiocytic granuloma of the tongue: case report. Br J Oral Maxillofac Surg 1991;29:350-2. [PubMed]
- Yong W. New recognition, classification and clinical features of histiocytic tumors. Oncology Progress 2006;4:152-6.
- Vassallo R, Ryu JH, Colby TV, et al. Pulmonary Langerhans’-cell histiocytosis. N Engl J Med 2000;342:1969-78. [PubMed]
- Murin S, Wiedemann HP, Matthay RA. Pulmonary manifestations of systemic lupus erythematosus. Clin Chest Med 1998;19:641-65. [PubMed]
- Shen M, Wang Y, Xu W, et al. Pleuropulmonary manifestations of systemic lupus erythematosus. SLE clinical manifestations of lung involvement. Chinese Medical Journal 2005;85:4.
- Haupt HM, Moore GW, Hutchins GM. The lung in systemic lupus erythematosus. Analysis of the pathologic changes in 120 patients. Am J Med 1981;71:791-8. [PubMed]