Staged percutaneous coronary intervention followed by minimally invasive mitral valve surgery versus combined coronary artery bypass graft and mitral valve surgery for two-vessel coronary artery disease and moderate to severe ischemic mitral regurgitation
Introduction
Moderate to severe ischemic mitral regurgitation (IMR) develops in 35% of patients after a myocardial infarction, and is associated with a 2-fold increase in mortality (1,2). While myocardial revascularization may improve IMR, there is a 40% incidence of persistent or worsening IMR at follow-up (3). Given the surgical risk associated with combined coronary artery bypass graft and mitral valve surgery (CABG+MVS), a staged procedure utilizing percutaneous coronary intervention followed by minimally invasive mitral valve surgery (PCI+MIVS) may be an alternative for concomitant coronary artery disease (CAD) and IMR (4). Staged procedures, also commonly referred to as “hybrid” approaches, have been associated with a lower morbidity and shorter hospital length of stay, when compared with conventional CABG and valve surgery, with reported 1- and 4.5-year survival rates of 92% and 88%, respectively (5-7). In the present study, we compared the results of PCI+MIVS with conventional CABG+MVS, in a non-randomized, consecutive series of patients with concomitant two-vessel CAD and moderate to severe IMR.
Methods
This study was approved by the Mount Sinai Medical Center Institutional Review Board. Our institutional Society of Thoracic Surgeons (STS) Database was retrospectively reviewed to identify patients with concomitant two-vessel CAD and moderate to severe IMR who underwent PCI+MIVS or CABG+MVR between February 2009 and April 2014. IMR was defined as new mitral regurgitation present
The baseline, echocardiographic, PCI, operative, and post-operative data of all patients was reviewed. The hospital length of stay for the PCI+MIVS group included the time allotted for the PCI. Vital status was assessed by reviewing local electronic health records, outpatient follow-up visits, and/or the Social Security Death Index. The definitions and variables selected were based on the society of thoracic surgeons (STS) Database definitions, version 2.73.
Patient selection and technique for PCI+MIVS
Patients were selected for a staged approach based on a suitable coronary anatomy amenable to PCI, with the consideration of co-morbidity and patient preference, after which the interventional cardiologist proceeded with PCI of all the angiographically or hemodynamically significant lesions. A loading dose of 600 mg of clopidogrel and 325 mg aspirin was administered at the time of PCI, followed by clopidogrel 75 mg and aspirin 81 to 325 mg daily, thereafter. The patients were continued on their anti-platelet therapy up to the day of MIVS, which was resumed on day 1 or 2 post-operatively.
Our approach to minimally invasive mitral valve surgery involves access via a right lateral thoracotomy, and has been previously described in detail (10). Briefly, the operation was performed via a 5–6 cm right thoracotomy, in the 4th or 5th intercostal space, lateral to the anterior axillary line. In patients undergoing mitral valve repair, the size of the anterior leaflet was used to determine the size of the annuloplasty ring implanted. Mitral valve replacement consisted of either excision of the anterior leaflet with preservation of the posterior leaflet and chords, or preservation of both leaflets. In two cases of significant leaflet tethering and papillary muscle displacement, the papillary muscles were approximated at their respective bases, utilizing a 4 mm polytetrafluoroethylene graft, as an adjunctive subvalvular repair (11).
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation (SD), or median and interquartile range (IQR, 25-75%). An independent t-test was utilized to compare continuous variables with a normal distribution, and a Mann-Whitney U test was used for variables without a normal distribution. Dichotomous variables were compared using a Fisher’s exact or Chi-square test, as appropriate. A two-tailed P value <0.05 was considered statistically significant. All statistical analyses were conducted using Statistical Package for Social Sciences, version 21 (SPSS Inc., Chicago, IL, USA).
Results
A total of 259 PCI+MIVS were identified, of which 9 had two-vessel CAD and moderate to severe IMR. During this time, there were 1,245 CABG operations performed, of which 15 had concomitant two-vessel CABG+MVS for moderate to severe IMR. The baseline characteristics between the groups were similar, except for a greater prevalence of pre-operative clopidogrel administration (78% versus 27%, P=0.03) and left anterior descending plus left circumflex CAD (78% versus 27%, P=0.03), in patients undergoing PCI+MVS as compared with CABG+MVS. The most common diseased coronary vessels amongst the CABG+MVS group were left anterior descending plus right CAD (40%), and left main plus right CAD (33%) (Table 1).
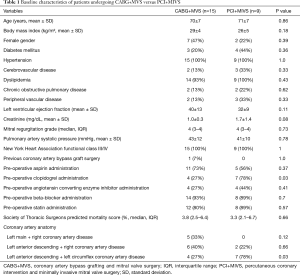
Full table
The median time from PCI to MIVS was 40 days (IQR, 8–71). All patients undergoing CABG+MVS received a left internal mammary artery to the left anterior descending coronary artery graft, with the mean number of total bypass grafts being 2.5±0.5. In patients who underwent PCI+MIVS, the mean number of coronary stents placed was 2.3±1, with drug-eluting stents utilized in 6 (67%). PCI+MIVS was associated with decreased mean cardiopulmonary bypass (111±41 versus 167±49 min, P=0.01) and aortic cross-clamp (79±32 versus 129±35 min, P=0.003) times, and fewer intraoperative packed red blood transfusions [2 (IQR, 0–2) versus 3 units (IQR, 1–4), P=0.05], when compared with CABG+MVS. The rate of mitral valve repair, postoperative complications, 30-day mortality, and 1-year survival did not differ between the surgical approaches (Table 2).
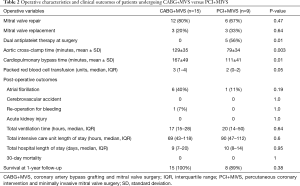
Full table
Discussion
Patients who undergo combined CABG+MVS are at higher surgical risk, with operative mortality rates of 4.1% to 9.8%, and major morbidity occurring in 7.0% to 11.6% (12). Thus, it is hypothesized that a hybrid approach of PCI+MIVS for concomitant CAD and moderate to severe IMR may reduce the operative risk by permitting the use of two smaller, less invasive procedures. Performing PCI for revascularization permits the use of minimally invasive surgical techniques, which have been demonstrated to reduce post-operative atrial fibrillation, wound infection, and the need for blood products, while decreasing surgical trauma and enhancing post-operative recovery (13,14). In the present study, PCI+MIVS was associated with shorter operative times and fewer intra-operative blood transfusions, as compared with CABG+MVS, in patients with two-vessel CAD and moderate to severe IMR.
When performed for secondary MR, a minimally invasive approach has been associated with a 30-day mortality rate of 2.1% to 2.8%, with valve repair being achieved in the majority of patients (10,15). Indeed, one of the advantages of using a right lateral thoracotomy approach to mitral valve surgery is the more en face view of the mitral apparatus, which allows for a clear delineation of the valvular anatomy (16). A minimally invasive right lateral thoracotomy has also been used for complex mitral valve repair, consisting of combined papillary muscle approximation and ring annuloplasty, in patients with moderate to severe secondary MR and advanced left ventricular remodeling (11,17). Finally, despite a higher risk profile, minimally invasive mitral valve surgery in patients with left ventricular systolic dysfunction can be performed with similar outcomes in regards to perioperative morbidity and postoperative recovery, as compared with patients with a normal left ventricular ejection fraction (15).
A concern with a hybrid approach is the risk of bleeding during valve surgery in patients post-PCI that are taking clopidogrel. In the present study, even though there was a higher use of pre-operative clopidogrel in those undergoing PCI+MIVS, there were fewer intraoperative transfusions required, when compared with CABG+MVS. The lower need for blood products in the PCI+MIVS group is most likely due to the fact that, by it less traumatic nature, minimally invasive valve surgery is associated with less blood loss. Also, by virtue of the fact that there was no need to place bypass grafts, the operative times were much shorter in this group, thereby having less bleeding (18). Importantly, there were no cases of acute stent thrombosis peri-operatively. In our previous work, we evaluated 222 patients who had PCI+MIVS, 183 of which were on clopidogrel and were compared with 38 who were not (6). In the intra-operative period, there were no differences in the requirement of blood products between the two groups. Post-operatively, there was a higher proportion of patients on clopidogrel requiring blood products compared with those who did not take it (50.5% versus 26.3%, P=0.005); however, there was no significant difference in the need for re-operation for bleeding. Because clopidogrel use peri-operatively appears to be safe, our clinical practice has been to continue anti-platelet therapy at the time of valve surgery to minimize the risk of acute stent thrombosis.
There are several limitations to the current study. Firstly, it is a single center, retrospective study of a highly selected, heterogeneous group of patients. Secondly, the patients were selected to undergo PCI+MIVS based on suitable coronary artery anatomy, which is an important selection bias. Thirdly, the small sample size provided inadequate statistical power to fully assess potential differences in baseline demographics and clinical outcomes between the groups. Fourthly, while all patients had two-vessel CAD, the higher proportion of left anterior descending plus left circumflex CAD in the PCI+MIVS introduces a potential uncontrollable confounder. Finally, the follow-up outcomes were limited to survival at 1-year of clinical follow-up, with data regarding target vessel revascularization unavailable without scheduled angiographic follow-up for the PCI+MIVS, which is an important marker of procedural success. Thus, our results are best viewed as hypothesis generating for future randomized trials comparing PCI+MIVS versus CABG+MVS in this higher-risk population.
In conclusion, a hybrid approach of PCI+MIVS for two-vessel CAD and moderate to severe IMR is feasible, and associated with satisfactory outcomes, as compared with traditional CABG+MVS. Future studies will better define the role and optimal timing of staged PCI and MIVS.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was approved by the Institutional Review Board at the Mount Sinai Medical Center, Miami Beach, Florida, USA.
References
- Magne J, Sénéchal M, Dumesnil JG, et al. Ischemic mitral regurgitation: a complex multifaceted disease. Cardiology 2009;112:244-59. [Crossref] [PubMed]
- Asgar AW, Mack MJ, Stone GW. Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. J Am Coll Cardiol 2015;65:1231-48. [Crossref] [PubMed]
- Kang DH, Sun BJ, Kim DH, et al. Percutaneous versus surgical revascularization in patients with ischemic mitral regurgitation. Circulation 2011;124:S156-62. [Crossref] [PubMed]
- Mihos CG, Santana O, Pineda AM, et al. Percutaneous coronary intervention followed by minimally invasive mitral valve surgery in ischemic mitral regurgitation. Innovations (Phila) 2015;10:394-7. [Crossref] [PubMed]
- Santana O, Funk M, Zamora C, et al. Staged percutaneous coronary intervention and minimally invasive valve surgery: results of a hybrid approach to concomitant coronary and valvular disease. J Thorac Cardiovasc Surg 2012;144:634-9. [Crossref] [PubMed]
- Santana O, Pineda AM, Cortes-Bergoderi M, et al. Hybrid approach of percutaneous coronary intervention followed by minimally invasive valve operations. Ann Thorac Surg 2014;97:2049-55. [Crossref] [PubMed]
- Santana O, Singla S, Mihos CG, et al. Outcomes of a combined approach of percutaneous coronary revascularization and cardiac valve surgery. Innovations (Phila) 2017;12:4-8. [Crossref] [PubMed]
- Borger MA, Alam A, Murphy PM, et al. Chronic ischemic mitral regurgitation: repair, replace or rethink? Ann Thorac Surg 2006;81:1153-61. [Crossref] [PubMed]
- Carpentier A. Cardiac valve surgery--the "French correction". J Thorac Cardiovasc Surg 1983;86:323-37. [PubMed]
- Santana O, Reyna J, Pineda AM, et al. Outcomes of minimally invasive mitral valve surgery in patients with an ejection fraction of 35% or less. Innovations (Phila) 2013;8:1-5. [Crossref] [PubMed]
- Mihos CG, Capoulade R, Yucel E, et al. Combined papillary muscle sling and ring annuloplasty for moderate-to-severe secondary mitral regurgitation. J Card Surg 2016;31:664-71. [Crossref] [PubMed]
- D'Agostino RS, Jacobs JP, Badhwar V, et al. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2017 Update on Outcomes and Quality. Ann Thorac Surg 2017;103:18-24. [Crossref] [PubMed]
- Schmitto JD, Mokashi SA, Cohn LH. Minimally-invasive valve surgery. J Am Coll Cardiol 2010;56:455-62. [Crossref] [PubMed]
- Goldstone AB, Joseph Woo Y. Minimally invasive surgical treatment of valvular heart disease. Semin Thorac Cardiovasc Surg 2014;26:36-43. [Crossref] [PubMed]
- Atluri P, Woo YJ, Goldstone AB, et al. Minimally invasive mitral valve surgery can be performed with optimal outcomes in the presence of left ventricular dysfunction. Ann Thorac Surg 2013;96:1596-601; discussion 1601-2. [Crossref] [PubMed]
- Langer NB, Argenziano M. Minimally Invasive Cardiovascular Surgery: Incisions and Approaches. Methodist Debakey Cardiovasc J 2016;12:4-9. [Crossref] [PubMed]
- Santana O, Solenkova NV, Pineda AM, et al. Minimally invasive papillary muscle sling placement during mitral valve repair in patients with functional mitral regurgitation. J Thorac Cardiovasc Surg 2014;147:496-9. [Crossref] [PubMed]
- Salis S, Mazzanti VV, Merli G, et al. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J Cardiothorac Vasc Anesth 2008;22:814-22. [Crossref] [PubMed]


