Predictive factors in the evaluation of treatment response to neoadjuvant chemoradiotherapy in patients with advanced esophageal squamous cell cancer
Introduction
The results of surgical resection of esophageal cancer have improved and mortality rate after esophagectomy is less than 5% in dedicated centers around the world. Long-term prognosis however remains suboptimal. Multimodality management strategies have been tested in order to improve outcome. Different regimens of multimodal therapies were explored in the last three decades involving pre- and post-operative chemotherapy and/or radiotherapy, with improved survival outcomes (1). Their adoption is widespread.
Since its publication in 2012, the Dutch Chemoradiotherapy for Oesophageal Cancer followed by Surgery Study (CROSS) has become a standard-of-care treatment for stage II and III esophageal cancer, both for squamous cell cancers and adenocarcinomas (2,3). It was a randomized controlled trial that involved 368 patients including both squamous and adenocarcinoma of the esophagus and gastroesophageal junction. Patients were randomized into either surgery alone group (188 patients) or chemoradiotherapy (weekly administration of carboplatin and paclitaxel with concurrent radiotherapy 41.4 Gy) followed by surgery (171 patients). The majority (75%) of patients had adenocarcinoma and the tumors were located either at the distal esophagus (58%) or esophagogastric junction (24%). The updated long-term results with a median follow-up for surviving patients of 84.1 months (range 61.1 to 116.8 months) showed that the median overall survival of the neoadjuvant chemoradiotherapy group was 48.6 months and was 24 months in the surgery alone group. The effect on squamous cell carcinomas was particularly impressive; the median overall survival for patients was 81.6 months in the neoadjuvant chemoradiotherapy plus surgery group and 21.1 months in the surgery alone group. Significant benefits were also found for patients with adenocarcinomas, median survival was 43.2 months in the neoadjuvant chemoradiotherapy plus surgery group and 27.1 months in the surgery alone group. Better control was found in both locoregional and distant disease progression. When translated to 5-year overall survival it was 47% versus 33% respectively. The update also provided additional data on median progression-free survival of 37.7 months in the neoadjuvant chemoradiotherapy group and 16.2 months in the surgery alone group.
Other regimens are also used. In some countries, preoperative or peri-operative chemotherapy is preferred without addition of radiotherapy. In Japan, neoadjuvant chemotherapy with cisplatin and 5-fluourocil (5-FU) is the current standard in Japan, based on the randomized trial JCOG 9907, which compared preoperative vs. postoperative chemotherapy (4). In the United Kingdom, the MAGIC trial regimen is commonly used for adenocarcinoma of the gastroesophageal junction (5). Neoadjuvant chemoradiotherapy has also demonstrated a trend of survival advantage over neoadjuvant chemotherapy alone for adenocarcinomas of the esophagogastric junction in the German POET trial (6). Some on-going trials are eagerly anticipated; for squamous cell cancers, the NExT (JCOG 1009) study utilizes a three-group design compares conventional neoadjuvant cisplatin/5-FU regimen with intensified chemotherapy adding Docetaxel, and a third study group of concurrent radiotherapy with cisplatin and 5-FU (7). The Neo-AEGIS trial is a randomized trial of combined neoadjuvant and adjuvant chemotherapy (modified MAGIC regimen) versus neoadjuvant chemoradiation (CROSS) for adenocarcinoma of the esophagus and esophagogastric junction. This study includes 10 centers in Denmark, Ireland and United Kingdom, aiming to recruit 366 patients. The Neo-AEGIS Trial will have its estimated primary completion date in 2024 (8).
Whatever regimen is used, neoadjuvant therapy aims at improving survival by tumor downstaging, increasing the chance of R0 resection with negative margins, and in theory also treat micrometastases. Such therapies (especially with radiotherapy) however, may have disadvantages. Patient’s physiological status may deteriorate after during chemoradiotherapy. They may become immunocompromised; radiotherapy may induce pneumonitis and cardiotoxicity. The fibrosis after radiotherapy may obscure tissue planes and may make surgery more challenging. Other surgical complications such as anastomotic leakage, recurrent laryngeal nerve injury are also reported to be more common after chemoradiotherapy, especially when surgery is carried out for salvage rather than in the neoadjuvant setting. A systemic review analysed pooled data from 954 patients in 8 studies, 712 patients underwent chemoradiotherapy with neoadjuvant intent and 242 had definitive chemoradiotherapy followed by salvage surgery. Morbidity and mortality rates were significantly higher in the salvage group (9). In CROSS, the in-hospital mortality rate was acceptable at 4%. Although there was no significant difference in the occurrence of postoperative complications between the two treatment groups, the actual incidence of anastomotic leaks was high at 22–30%. The authors did not give any postulation or explanation to this phenomenon. This figure is approaching the complication rate in salvage esophagectomy after radical chemoradiotherapy. From the Nationwide database in Japan of 5,354 patients in 2011, the anastomotic leakage rate was 13.3% and the thirty-day mortality rate was 1.2% (10). At the authors’ institution, post-operative morbidity and hospital mortality rates are comparable for multimodal therapy and upfront surgery. Our experience with neoadjuvant chemoradiotherapy in squamous cell carcinoma demonstrated significant tumor downstaging in 75% of patients, pathological complete response occurred in 31% (11).
A significant minority of patients will not respond to neoadjuvant treatment with no added benefits or even harm as detailed above, and moreover surgical treatment is delayed. It is imperative that non-responders to treatment can be identified so that they are not exposed to potentially harmful chemoradiotherapy. Alternative strategies can be designed.
Histopathological assessment of response to neoadjuvant therapy and prediction of survival outcome
There are different means of measuring tumor response to neoadjuvant therapy. Clinically the Response Evaluation Criteria in Solid Tumor (RECIST) criteria are often used, measured radiologically. Ultimately the definitive assessment will be by histopathology in patients whose tumors are resected.
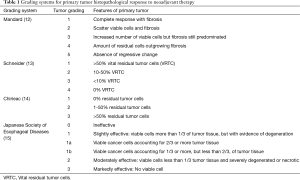
Mandard and associates investigated the pathological features of esophageal carcinoma in post-neoadjuvant chemoradiation esophagectomy specimens and were the first to report a prognostic classification using histopathological regression. They classified tumor regression into a scale of five, according to the degree of chemoradiation-induced fibrosis in relation to residual tumor cells (12). Schneider used a 4-category system of >50% vital residual tumor cells (VRTC), 10–50% VRTC, <10% VRTC, and 0% VRTC (13). The system by Chirieac classified responses into 0% residual tumor cells, 1-50% residual cells and more than 50% viable tumor cells (14). The classification by the Japanese Society for Esophageal Disease divided responses into class 0 as ineffective, class 1 as slightly effective with viable cells more than one third of tumor tissue with evidence of degeneration, class 2 as moderately effective with viable cells less than one third of tumor tissue and severely degenerated, and class 3 which is markedly effective with no viable cells left (15) (Table 1). Pathological regression rate has prognostic value, and has been repeatedly shown by investigators (12,14,16). The authors have reported that on multivariate analysis in 175 patients who had had neoadjuvant chemoradiotherapy followed by surgery, male gender, high percentage of residual viable tumor cells in the primary tumor, and positive nodal status were independent predictors of poor prognosis (11). In the literature, it has been shown consistently a good response to neoadjuvant therapies (especially pathological complete response pCR) is a favourable prognostic factor.
Full table
Predictive markers of histopathological response to neoadjuvant therapy
For all of its advantages, neoadjuvant chemoradiotherapy is a double-edged sword. Good responders may have better survival in the long term, but in poor responders, disease may progress during the course of neoadjuvant treatment. There is thus an urgent need to identify accurate predictors of response, so that patients are not exposed to potentially harmful treatments without benefits, and management strategy can be modified accordingly. Much effort has been made in this regard. Clinical, biochemical and molecular predictors were identified for tumor regression after neoadjuvant therapy.
Clinical predictors
18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) scans is widely used to stage esophageal cancer, and also to measure tumor activity. Standard uptake value (SUV) provides a measurement of viable tumor cell density. In a study by Ajani et al, post-chemoradiation SUV value of the primary tumor was an independent predictor of pathological complete response on multivariate analysis, in addition to several other clinical parameters (17). The study included both esophageal squamous cell carcinomas and adenocarcinomas. Female gender, well or moderate tumor differentiation, baseline T-stage by endoscopic ultrasound and absence of cancer cells on post-chemoradiation biopsies were positive predictors of pathological complete response. These factors were constructed into a normogram to predict pathological complete response. At a score of more than 160 points, the normogram predicted pCR with a probability of up to 60%, more reliably than any of the variables alone. Moreover, the pCR prediction normogram correlated with survival of patients who received definitive chemoradiotherapy (18). At the author’s institution, SUV value was also found to be a predictive factor. In 52 patients who had undergone neoadjuvant chemoradiotherapy and then surgical resection, 21 (40.4%) achieved pathological complete response (pCR). SUVmax of the primary tumor at one month post neoadjuvant therapy was independently predictive of pCR. However the predictive value was only modest, sensitivity was 71%, specificity was 66.7%, positive predictive value was 75.9%, and negative predictive value was 60.9% (19).
A meta-analysis assessed 20 studies concluded that SUV response on FDG-PET was not accurate enough to predict tumor response to neoadjuvant chemoradiotherapy (20). Using more stringent criteria to include studies of higher quality, another meta-analysis included 13 studies, found that the pooled sensitivity, specificity, and diagnostic odds ratios for FDG-PET in the evaluation of neoadjuvant therapy response was 70.3%, 70.1% and 9.389 respectively (21). However the criteria for response based on PET and histopathological response were variable and therefore firm conclusions may not be entirely satisfactory. It was also interesting to note that studies of chemotherapy alone did not differ significantly compared with those with chemoradiotherapy, in spite of an expectation that radiation induced esophagitis and inflammation might be a possible confounding factor in PET assessment.
The role of PET scan in early assessment of neoadjuvant response before the whole treatment is completed has been explored by some investigators. PET scan as early as two weeks into neoadjuvant chemotherapy was demonstrated to predict pathological complete response in adenocarcinomas of esophagus and esophagogastric junction. Its accuracy of prediction was similar to that of PET scan performed after completion of neoadjuvant therapy, perhaps implying steady histopathological response characteristics of adenocarcinomas (22). Similarly, a study was conducted in the context of squamous cell cancer of the esophagus. The absolute SUV levels at baseline and at two weeks into neoadjuvant chemoradiotherapy correlated with histopathological response. R0 resection and significantly better survival were achieved in patients with an SUV reduction of more than 30% (23). Then again the conclusion was drawn from a rather small number of patients. Whether this approach could be applied to predict pathological response at the early stage of neoadjuvant therapy for reliably needs to be elucidated in further studies.
Biochemical predictors
Blood parameters have been studied for prediction of tumor response to neoadjuvant therapy in esophageal and other cancers. Markers involved in inflammation and coagulation cascade appear to play a role in tumor progression but the precise mechanism is unclear.
Pre-treatment neutrophil-to-lymphocyte ratio (NLR) was proposed as a predictive marker for response to neoadjuvant chemotherapy in a Japanese study. Patients underwent the same neoadjuvant regime in the JCOG 9907 trial and the majority had esophageal squamous cell cancers. An NLR of less than 2.2 was statistically associated with pathological response. The threshold for pathological response in this study was low however, at one-third disappearance of tumor cells (24). This is likely because of a low response rate to neoadjuvant chemotherapy in this study, with over 60% having less than one-third tumor cell disappearance, compared to previously reported figures (11).
Plasma fibrinogen is postulated by some to promote tumor progression by adherence of circulating tumor cells to the vasculature of distant organs, and was shown in several studies to be associated with tumor recurrence and metastases (25,26). It also influences integrin expression on endothelial cells and possibly modulates cellular pathways in tumor progression. One study showed that a high pre-treatment plasma fibrinogen concentration, amongst other coagulative factors and acute phase proteins, was independently associated with good pathological response to neoadjuvant therapy. Eighty-four patients had either chemotherapy or chemoradiotherapy before surgical resection, the majority had adenocarcinoma (27). However this was contradicted by another Japanese study on esophageal squamous cell cancer patients treated with either upfront resection or radical chemoradiotherapy. Low pre-treatment plasma fibrinogen concentration was associated with complete response in a subgroup of stages II and III patients. Study patients however had definitive chemoradiation without surgery, therefore assessment of treatment response was based only on clinical grounds as resected specimens were not available. The selection criteria for treatment was also not clearly stated (26). Further investigations would be necessary to clarify the role and predictive value of fibrinogen.
The advantage of biochemical predictors is that they could be easily obtained from simple blood tests. No single marker however has been shown to be reliable so far.
Molecular predictors
With growing understanding of cancer genetics, certain genes appear to modulate tumor proliferation and chemotherapeutic drug metabolism. One of the most studied molecule is probably p53. It has been documented in the literature that p53, a tumor suppressor gene, enhances chemosensitivity. The exact mechanism of which p53 mediates neoadjuvant response awaits to be elucidated. A proposed mechanism theorizes that p53 mutation inhibits p73 that in turn mediates cellular apoptosis following Cisplatin exposure in head and neck squamous cell cancers (28).
Studies supported wild-type p53, identified from pre-treatment endoscopic biopsies, as a good prognosticator for esophageal cancer patients who underwent neoadjuvant chemotherapy. In a meta-analysis examining the correlation between p53 status and response to chemotherapy-based treatment, wild-type p53 gene exhibited an association with pathological complete response in esophageal squamous cell cancer patients who were treated with neoadjuvant chemotherapy (29). However, in the experience of the authors’ institution, the correlation between pathological response and p53 expression was not as robust (30).
Low level of excision-repair cross-contemplating 1 (ERCC1) mRNA expression has also been shown to link with superior response to Cisplatin-based chemotherapy in esophageal squamous cell cancers. It was demonstrated in a study that ERCC1 levels in esophageal squamous cancers with partial response to Cisplatin-based chemoradiotherapy were significantly lower than the levels in cancers without response, and that those with lower ERCC1 levels had greater sensitivity to platinum drugs (31).
Molecular processes in tumor biology are complex, pathways that are involved in cellular response to chemoradiotherapy are likely multistep and interlinked. Individual genes could pertain to only small fractions of the sequential cellular processes. This is a possible limitation in establishing single genes as predictors of response to neoadjuvant therapy.
Microarray studies possibly resolve this problem. The merit of microarray expression analysis is that it simultaneously detects several genetic markers that are differentially expressed in the pre-treatment tumor biopsies of responders and non-responders. Using the medley of detected genetic markers, logistic regression models could be generated to predict pathological response. Two studies proposed such prediction models using several differentially expressed single-stranded non-coding microRNAs; miR-145-5p, miR-152, miR-193-3p and miR-376a-3p were used in one study (32), and MMP1, LIMCH1 and Clorf226 in another (33). Both looked at esophageal squamous cell cancer patients who underwent a standardized neoadjuvant regime, and the prediction models had high probabilities. A 32-gene classifier was identified in another study using complementary DNA microarray analysis of pre-treatment esophageal cancer biopsies. A mixed cohort of adenocarcinoma and squamous cell carcinomas was recruited, but when applied to squamous cell histology alone (21 samples) the proposed multi-gene model was capable of predicting pathological response (34). Although further studies are required to verify these models as their patient numbers were small, it is apparent that neoadjuvant treatment response is an interplay between several genes.
The aforementioned studies were conducted on pre-treatment tumor biopsies. Recent studies have also demonstrated the predictive power of blood-based molecular markers. The pathological response prediction of ERCC1 was elicited in a study analysing pre-treatment serum samples of esophageal cancer patients who underwent Cisplatin-based neoadjuvant chemoradiation (35). A microarray study was conducted at the authors’ institution on peripheral blood samples of 20 esophageal squamous cell cancer patients who responded differently to Cisplatin- and 5-fluorouracil-based neoadjuvant chemoradiotherapy. Responders were defined as those with 0% viable tumor cells in resected tissues from esophagectomy specimens, while those with at least 50% residual viable tumor cells were classified as non-responders. Upon validation in a larger set of responders and non-responders, MiR-193b in peripheral blood was shown to correlate significantly with response (unpublished data). These studies shed light onto circulating biomarkers as potential non-invasive tools for better patient selection for neoadjuvant therapy.
Thanks to the rapidly-growing field of molecular medicine, certain genes have evolved as potential response predictors. Albeit the optimism, molecular analysis is costly and labour intensive. Applying these biomarkers to clinical practice is not without hurdles.
Limitations of current studies on predictive markers for neoadjuvant response
Most of the studies were conducted on small samples of patients. Since responders and non-responders were defined differently, and the neoadjuvant regimes and tumor cell types were heterogenous in the studies, data could not be easily pooled for analysis. In addition, it is impossible to distinguish between spontaneous tumor necrosis and neoadjuvant therapy induced tumor necrosis histologically. The predictive power of the proposed markers may after all be over- or under-estimated. Stronger evidence is still needed to validate these methods of neoadjuvant response prediction.
Response prediction and its implication on treatment for esophageal cancer
For patients with predicted pCR, neoadjuvant chemoradiotherapy is undoubtedly the treatment of choice. Much evidence has shown that those with pCR had better survival (36-39). How about patients with predicted subtotal pathological response? An unanswered question is, perhaps, what degree of histological tumor regression is required to bring upon a meaningful survival difference for patients. It is impossible to tell for the meantime, given the arbitrary definitions of pathological response used in current studies. In some studies, pathological responders were defined as pCR; in many others, it was defined in range of response percentages. This area will remain uncertain until this endpoint measurement is more clearly defined in future studies.
One of the latest areas of research is to test the concept of surveillance and salvage surgery only in the event of recurrence in predicted complete responders. This is being tested in an ongoing multicentre French trial (40). Another Dutch group is expected to conduct a randomized controlled trial (the SANO trial), to examine the safety of the same strategy, for those clinically assessed as complete responders. Prior to this trial, it has conducted a study to test the accuracy of clinical response evaluation in predicting pCR (the PreSANO study) (41). Study results are eagerly awaited.
Conclusions
Currently there is no reliable predictive marker of response to neoadjuvant chemoradiotherapy and is an active area for research. Continuing effort is required to fill the gaps of our understanding in the biology of chemoradiation response. Further studies are to be conducted to validate the potential markers, and to overcome problems of small sample size, non-standardized neoadjuvant regime and variable pathological response measurement. In the future, treatment for esophageal cancer hopefully will not only be individualized to disease stage and physical status of patients, but also be tailored to an accurately predicted response to chemoradiotherapy. The role of esophagectomy is evolving.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Law S, Kwong DL, Kwok KF, et al. Improvement in treatment results and long-term survival of patients with esophageal cancer: impact of chemoradiation and change in treatment strategy. Ann Surg 2003;238:339-47; discussion 347-8. [PubMed]
- Shapiro J, Van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Van Hagen P, Hulshof M, Van Lanschot J, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907) Ann Surg Oncol 2012;19:68-74. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009;27:851-6. [Crossref] [PubMed]
- Nakamura K, Kato K, Igaki H, et al. Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study) Jpn J Clin Oncol 2013;43:752-5. [Crossref] [PubMed]
- Keegan N, Keane F, Cuffe S, et al. ICORG 10-14:Neo-AEGIS:A randomized clinical trial of neoadjuvant and adjuvant chemotherapy(modified MAGIC regimen)versus neoadjuvant chemoradiation(CROSS protocol)in adenocarcinoma of the esophagus and esophagogastric junction, 2014.
- Markar SR, Karthikesalingam A, Penna M, et al. Assessment of short-term clinical outcomes following salvage esophagectomy for the treatment of esophageal malignancy: systematic review and pooled analysis. Ann Surg Oncol 2014;21:922-31. [Crossref] [PubMed]
- Takeuchi H, Miyata H, Gotoh M, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg 2014;260:259-66. [Crossref] [PubMed]
- Tong DK, Law S, Kwong DL, et al. Histological regression of squamous esophageal carcinoma assessed by percentage of residual viable cells after neoadjuvant chemoradiation is an important prognostic factor. Ann Surg Oncol 2010;17:2184-92. [Crossref] [PubMed]
- Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994;73:2680-6. [Crossref] [PubMed]
- Schneider PM, Baldus SE, Metzger R, et al. Histomorphologic tumor regression and lymph node metastases determine prognosis following neoadjuvant radiochemotherapy for esophageal cancer: implications for response classification. Ann Surg 2005;242:684-92. [Crossref] [PubMed]
- Chirieac LR, Swisher SG, Ajani JA, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer 2005;103:1347-55. [Crossref] [PubMed]
- Japan Esophageal S. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus 2017;14:1-36.
- Langer R, Ott K, Feith M, et al. Prognostic significance of histopathological tumor regression after neoadjuvant chemotherapy in esophageal adenocarcinomas. Mod Pathol 2009;22:1555-63. [Crossref] [PubMed]
- Ajani JA, Correa AM, Hofstetter WL, et al. Clinical parameters model for predicting pathologic complete response following preoperative chemoradiation in patients with esophageal cancer. Ann Oncol 2012;23:2638-42. [Crossref] [PubMed]
- Lin SH, Wang J, Allen PK, et al. A nomogram that predicts pathologic complete response to neoadjuvant chemoradiation also predicts survival outcomes after definitive chemoradiation for esophageal cancer. J Gastrointest Oncol 2015;6:45-52. [PubMed]
- Yuan H, Tong DK, Vardhanabhuti V, et al. PET/CT in the evaluation of treatment response to neoadjuvant chemoradiotherapy and prognostication in patients with locally advanced esophageal squamous cell carcinoma. Nucl Med Commun 2016;37:947-55. [Crossref] [PubMed]
- Kwee RM. Prediction of tumor response to neoadjuvant therapy in patients with esophageal cancer with use of 18F FDG PET: a systematic review. Radiology 2010;254:707-17. [Crossref] [PubMed]
- Chen YM, Pan XF, Tong LJ, et al. Can 18F-fluorodeoxyglucose positron emission tomography predict responses to neoadjuvant therapy in oesophageal cancer patients? A meta-analysis. Nucl Med Commun 2011;32:1005-10. [Crossref] [PubMed]
- Wieder HA, Ott K, Lordick F, et al. Prediction of tumor response by FDG-PET: comparison of the accuracy of single and sequential studies in patients with adenocarcinomas of the esophagogastric junction. Eur J Nucl Med Mol Imaging 2007;34:1925-32. [Crossref] [PubMed]
- Wieder HA, Brücher BL, Zimmermann F, et al. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol 2004;22:900-8. [Crossref] [PubMed]
- Sato H, Tsubosa Y, Kawano T. Correlation between the pretherapeutic neutrophil to lymphocyte ratio and the pathologic response to neoadjuvant chemotherapy in patients with advanced esophageal cancer. World J Surg 2012;36:617-22. [Crossref] [PubMed]
- Matsuda S, Takeuchi H, Fukuda K, et al. Clinical significance of plasma fibrinogen level as a predictive marker for postoperative recurrence of esophageal squamous cell carcinoma in patients receiving neoadjuvant treatment. Dis Esophagus 2014;27:654-61. [Crossref] [PubMed]
- Takeuchi H, Ikeuchi S, Kitagawa Y, et al. Pretreatment plasma fibrinogen level correlates with tumor progression and metastasis in patients with squamous cell carcinoma of the esophagus. J Gastroenterol Hepatol 2007;22:2222-7. [Crossref] [PubMed]
- Ilhan-Mutlu A, Starlinger P, Perkmann T, et al. Plasma fibrinogen and blood platelet counts are associated with response to neoadjuvant therapy in esophageal cancer. Biomark Med 2015;9:327-35. [Crossref] [PubMed]
- Bergamaschi D, Gasco M, Hiller L, et al. p53 polymorphism influences response in cancer chemotherapy via modulation of p73-dependent apoptosis. Cancer Cell 2003;3:387-402. [Crossref] [PubMed]
- Zhang SS, Huang QY, Yang H, et al. Correlation of p53 status with the response to chemotherapy-based treatment in esophageal cancer: a meta-analysis. Ann Surg Oncol 2013;20:2419-27. [Crossref] [PubMed]
- Lam KY, Law S, Ma LT, et al. Pre-operative chemotherapy for squamous cell carcinoma of the oesophagus: do histological assessment and p53 overexpression predict chemo-responsiveness? Eur J Cancer 1997;33:1221-5. [Crossref] [PubMed]
- Tanaka K, Mohri Y, Ohi M, et al. Excision-repair cross-complementing 1 predicts response to cisplatin-based neoadjuvant chemoradiotherapy in patients with esophageal squamous cell carcinoma. Mol Med Rep 2009;2:903-9. [PubMed]
- Wen J, Luo K, Liu H, et al. MiRNA expression analysis of pretreatment biopsies predicts the pathological response of esophageal squamous cell carcinomas to neoadjuvant chemoradiotherapy. Ann Surg 2016;263:942-8. [Crossref] [PubMed]
- Wen J, Yang H, Liu MZ, et al. Gene expression analysis of pretreatment biopsies predicts the pathological response of esophageal squamous cell carcinomas to neo-chemoradiotherapy. Ann Oncol 2014;25:1769-74. [Crossref] [PubMed]
- Duong C, Greenawalt DM, Kowalczyk A, et al. Pretreatment gene expression profiles can be used to predict response to neoadjuvant chemoradiotherapy in esophageal cancer. Ann Surg Oncol 2007;14:3602-9. [Crossref] [PubMed]
- Brabender J, Vallböhmer D, Grimminger P, et al. ERCC1 RNA expression in peripheral blood predicts minor histopathological response to neoadjuvant radio-chemotherapy in patients with locally advanced cancer of the esophagus. J Gastrointest Surg 2008;12:1815-21. [Crossref] [PubMed]
- Forastiere AA, Orringer MB, Perez-Tamayo C, et al. Preoperative chemoradiation followed by transhiatal esophagectomy for carcinoma of the esophagus: final report. J Clin Oncol 1993;11:1118-23. [Crossref] [PubMed]
- Swisher SG, Ajani JA, Komaki R, et al. Long-term outcome of phase II trial evaluating chemotherapy, chemoradiotherapy, and surgery for locoregionally advanced esophageal cancer. Int J Radiat Oncol Biol Phys 2003;57:120-7. [Crossref] [PubMed]
- Geh JI, Crellin AM, Glynne-Jones RP. Chemoradiotherapy in oesophageal cancer. Inpharma Weekly 1999;1187:16.
- Ancona E, Ruol A, Santi S, et al. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma - Final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer 2001;91:2165-74. [Crossref] [PubMed]
- Comparison of systemic surgery versus surveillance and rescue surgery in operable oesophageal cancer with a complete clinical response to radiochemotherapy (esostrate). Available online: https://clinicaltrials.gov/ct2/show/NCT02551458
- Noordman BJ, Shapiro J, Spaander MC, et al. Accuracy of Detecting Residual Disease After Cross Neoadjuvant Chemoradiotherapy for Esophageal Cancer (preSANO Trial): Rationale and Protocol. JMIR Res Protoc 2015;4:e79. [Crossref] [PubMed]


