Lung ultrasound: a new basic, easy, multifunction imaging diagnostic tool in children undergoing pediatric cardiac surgery
In their article entitled “Lung ultrasound profile after cardiopulmonary bypass in paediatric cardiac surgery: first experience in a simple cohort” (1) recently published on the Interactive Cardiovascular and Thoracic Surgery Journal, Vitale V. and colleagues discussed the incidence and the degree of pulmonary congestion in 20 neonates and infants (median age 3.25, inter quartile range 3.0–7.25 months) after pediatric cardiac surgery. Lung ultrasound (LUS) examinations were performed at 0, 1 and 2 post-operative days. The authors divided the thorax into four major scanning areas (1) (right and left apex and right and left bases) and identified three different profiles of lung congestion based on a previously classification reported by Raimondi and colleagues (2). The profile A (white lung), was defined as the presence of confluent B lines in two or more of the four areas, profile B as the prevalence of B lines in two or more of the scanned area and profile C (no congestion, normal lung) as the prevalence of A lines.
Vitale et al. (1) investigated the associations among LUS profiles and cardiopulmonary bypass (CPB) duration, aortic clamp time and markers of post-operative outcome including time of extubation and intensive care unit (ICU) stay. As expected, patients with B and C profile had longer CPB and cross clamp time, longer need of mechanical ventilation and higher stay in ICU. In particular these differences were significant at examination performed at first post-operative day (1).
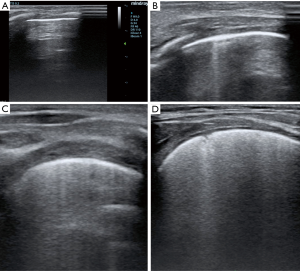
Of interest, authors employed a classification to grade lung congestion different from standardized adults protocols (3,4), grading B lines from 0 to 100% and disease severity from none to severe (3,4). In adult patients, a percentage of B lines ≤5% indicates absent pulmonary congestion, from 6% to 15% mild, 16–30% moderate, and >30% severe (3,4). The presence of B lines indicates a loss of air per volume of lung tissue with a correspondent increase of extra-vascular lung water (EVLW). In adults a normal LUS examination corresponds to a minimal presence of EVLW (i.e. <5% or <500 mL), while findings indicative for a severe congestion may indicate an overt pulmonary oedema (i.e., EVLW >90% or >2,000 mL) (5). In children there is not a standardized system to classify pulmonary congestion according to the percentage of B lines. The classification proposed by Raimondi and colleagues (2) and applied in the work of Vitale et al. (1) is an interesting simplification of the adult scoring system, but by diving only in three categories it may represent an over-simplification. In particular if the extremities (none and severe) of this score seems to be reasonable, there is no differentiation among mild to moderate grade of lung congestion (Figure 1). Authors (1) also limited the examination to the anterior and lateral approach, while the posterior approach has not been performed, probably for the difficulty to turn unstable patients with multiple wounds and drainage. According to standardized protocols (3,4) for each hemi-thorax three major areas (anterior, lateral and posterior) delineated by the para-sternal, anterior axillary and posterior axillary line should be identified. Every area may be further divided into the upper and lower half. Thus for a complete LUS examination (3,4), six different quadrants for each hemithorax should be scanned (anterior superior, anterior inferior, lateral superior, lateral inferior, posterior superior, posterior inferior).
Pulmonary complications are common in pediatric patients after cardiac surgery, and LUS may allow for fast diagnosis at patient’s bed (5-8), and monitoring lung disease progression and/or the response to medical therapy (i.e., diuretics) and physiotherapy (5-8). Despite this, the use of LUS in pediatric cardiac surgery remains very limited (6).
The utility of LUS in neonatal ICU has been proved by several authors (9-13). In contrast only a few small works evaluated potentialities of LUS in children undergoing cardiac surgery (5-8). There is multiple potential application of LUS in pediatric cardiac surgery. LUS may be employed for the diagnosis of many common lung complications occurring after cardiac surgery including atelectasis, effusion, lung congestion, pneumonia, pneumothorax and diaphragmatic motion anomalies (5-8). In contrast to X-RAY, LUS may allow to differentiate among effusion and atelectasis that are very common sequelae of cardiac surgery which require different therapeutic approaches (5-8,14). Our group (5) recently described a new potential application of chest ultrasound in the diagnosis of retro-sternal clots after pediatric cardiac surgery, that are common cause of hemodynamic unbalance and often difficult to diagnosis with conventional echocardiography. LUS may also guide interventional procedures such as drainage insertion for pleural effusion and pneumothorax (8,15). Large studies conducted in adults have shown how the routine use of LUS may drastically reduce (i.e., from 8.8% to 0.97%, P<0.0001) the risk of pneumothorax in thoracentesis (15). LUS may be used also to monitor the occurrence of pneumothorax after drainage removal, avoiding the repetition of chest X-RAY examinations (that is a routine practice in many Centers) (6,15). Utility of LUS has been proved also for tracheal tube verification in NICU (14). A recent review (14) on the accuracy and feasibility of LUS for tracheal tube placement in children reported how direct visualization of tracheal tube tip was highly feasible (i.e., 83% to 100%) in 165 cases evaluated from nine different studies. LUS held a high sensitivity (i.e., 1.00) for tracheal placement versus oesophageal placement, but only one study reported oesophageal intubation (i.e., specificity of 1.00). When assessing the appropriate tracheal tube depth for tracheal intubation using LUS, LUS also showed a good sensitivity (i.e., 0.91 to 1.00) and sufficient specificity (i.e., 5 to 1.0) for appropriate tracheal tube depth verification. Vitale and colleagues (1) focus their attention on prognostic potentialities of LUS findings, showing how the degree of lung congestion may correlate with intubation and ICU time. Data on prognostic utility of LUS in children are very limited. In adults instead (5) the prognostic value of lung water has been proved in different setting including heart failure (16), acute coronary syndrome (17,18) and dialysis (19). Furthermore the persistence of pulmonary congestion predicts re-hospitalization in heart failure (20,21).
LUS is a relatively easy technique that requires a short period of training. There has been a great debate on who should perform LUS. Theoretically LUS should be performed by the physician who is in charge of the patient (anaesthetist, cardiologist, surgeon). Some recent works (22,23) suggest how the performance of LUS should be extended to other professional skills including physiotherapist (22) and nurses (23). Particularly for physiotherapist LUS may represent a unique tool to guide their treatment and monitor their results (22).
LUS is an easy, accurate, fast, cheap and radiation free tool for the diagnosis and follow up of pulmonary complications in pediatric cardiac surgery. The use of LUS should be encourage to avoid the repetition of chest X-ray examination, that are expensive, and expose the children to cumulative and potentially dangerous dose of radiations (24). LUS furthermore may guide some common interventional manoeuvres (i.e., drainage insertion, tracheal tube verification, lung recruitment, etc.).
In summary, potentialities of LUS in pediatric cardiac surgery setting so far have been surprisingly underestimated, and its use extremely limited. As demonstrated by Vitale and colleagues (1), LUS may provide not only diagnostic but also prognostic information in pediatric cardiac surgery setting. LUS should become a basic diagnostic tool for multiple professional skills involved in the care of the children undergoing cardiac surgery for CHD including cardiologist, anaesthetists, surgeons, physiotherapist (22) and nurses (23). New research are warranted in order to evaluate potentialities and limitations of LUS in children undergoing cardiac surgery for CHD, as well as to establish classification system to evaluate disease severity (i.e., systems to classify lung congestion by B lines, etc.) and to assess prognostic potentialities of LUS findings.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vitale V, Ricci Z, Gaddi S, et al. Lung ultrasound profile after cardiopulmonary bypass in paediatric cardiac surgery: first experience in a simple cohort. Interact Cardiovasc Thorac Surg 2017;24:598-602. [PubMed]
- Raimondi F, Migliaro F, Sodano A, et al. Can neonatal ultrasound monitor fluid clearence and preditc the need of respiratory support? Crit Care 2012;16:R220. [Crossref] [PubMed]
- Volpicelli G, Elbarbary M, Blaivas M, et al. International Liaison Committee on Lung Ultrasound (ILC-LUS) for Interna tional Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012;38:577-91. [Crossref] [PubMed]
- Picano E, Frassi F, Agricola E, et al. Ultrasound lung comets: a clinically useful sign of extravascular lung water. J Am Soc Echocardiogr 2006;19:356-63. [Crossref] [PubMed]
- Cantinotti M, Giordano R, Assanta N, et al. Chest Ultrasound: A New, Easy, and Radiation-Free Tool to Detect Retrosternal Clot After Pediatric Cardiac Surgery. J Cardiothorac Vasc Anesth 2015;29:e59-60. [Crossref] [PubMed]
- Cantinotti M, Giordano R, Volpicelli G, et al. Lung ultrasound in adult and paediatric cardiac surgery: is it time for routine use? Interact Cardiovasc Thorac Surg 2016;22:208-15. [Crossref] [PubMed]
- Vitale V, Ricci Z, Cogo P. Lung ultrasonography and pediatric cardiac surgery: first experience with a new tool for postoperative lung complications. Ann Thorac Surg 2014;97:e121-4. [Crossref] [PubMed]
- Polito A, Biasucci DG, Cogo P. Point-of-care pleural and lung ultrasound in a newborn suffering from cardiac arrest due to tension pneumothorax after cardiac surgery. Cardiol Young 2016;26:400-2. [Crossref] [PubMed]
- Conlon TW, Himebauch AS, Fitzgerald JC, et al. Implementation of a pediatric critical care focused bedside ultrasound training program in a large academic PICU. Pediatr Crit Care Med 2015;16:219-26. [Crossref] [PubMed]
- Chen SW, Fu W, Liu J, et al. Routine application of lung ultrasonography in the neonatal intensive care unit. Medicine (Baltimore) 2017;96:e5826. [Crossref] [PubMed]
- Claes AS, Clapuyt P, Menten R, et al. Performance of chest ultrasound in pediatric pneumonia. Eur J Radiol 2017;88:82-87. [Crossref] [PubMed]
- Lin MJ, Gurley K, Hoffmann B. Bedside Ultrasound for Tracheal Tube Verification in Pediatric Emergency Department and ICU Patients: A Systematic Review. Pediatr Crit Care Med 2016;17:e469-e476. [Crossref] [PubMed]
- Liu J, Ren XL, Fu W, et al. Bronchoalveolar lavage for the treatment of neonatal pulmonary atelectasis under lung ultrasound monitoring. J Matern Fetal Neonatal Med 2016.1-5. [Epub ahead of print]. [PubMed]
- Soldati G, Smargiassi A, Inchingolo R, et al. Ultrasound-guided pleural puncture in supine or recumbent lateral position - feasibility study. Multidiscip Respir Med 2013;8:18. [Crossref] [PubMed]
- Cavanna L, Mordenti P, Bertè R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol 2014;12:139. [Crossref] [PubMed]
- Platz E, Lewis EF, Uno H, et al. Detection and prognostic value of pulmonary congestion by lung ultrasound in ambulatory heart failure patients. Eur Heart J 2016;37:1244-51. [Crossref] [PubMed]
- Frassi F, Gargani L, Tesorio P, et al. Prognostic value of extravascular lung water assessed with ultrasound lung comets by chest sonography in patients with dyspnea and/or chest pain. J Card Fail 2007;13:830-5. [Crossref] [PubMed]
- Bedetti G, Gargani L, Sicari R, et al. Comparison of prognostic value of echographic [corrected] risk score with the Thrombolysis in Myocardial Infarction (TIMI) and Global Registry in Acute Coronary Events (GRACE) risk scores in acute coronary syndrome. Am J Cardiol 2010;106:1709-16. [Crossref] [PubMed]
- Zoccali C, Torino C, Tripepi R, et al. Lung US in CKD Working Group. Pulmonary congestion predicts cardiac events and mortality in ESRD. J Am Soc Nephrol 2013;24:639-46. [Crossref] [PubMed]
- Gargani L, Pang PS, Frassi F, et al. Persistent pulmonary congestion before discharge predicts rehospitalization in heart failure: a lung ultrasound study. Cardiovasc Ultrasound 2015;13:40. [Crossref] [PubMed]
- Coiro S, Rossignol P, Ambrosio G, et al. Prognostic value of residual pulmonary congestion at discharge assessed by lung ultrasound imaging in heart failure. Eur J Heart Fail 2015;17:1172-81. [Crossref] [PubMed]
- Leech M, Bissett B, Kot M, et al. Lung ultrasound for critical care physiotherapists: a narrative review. Physiother Res Int 2015;20:69-76. [Crossref] [PubMed]
- Vitale J, Mumoli N, Giorgi-Pierfranceschi M, et al. Comparison of the Accuracy of Nurse-Performed and Physician-Performed Lung Ultrasound in the Diagnosis of Cardiogenic Dyspnea. Chest 2016;150:470-1. [Crossref] [PubMed]
- Ait-Ali L, Andreassi MG, Foffa I, et al. Cumulative patient effective dose and acute radiation-induced chromosomal DNA damage in children with congenital heart disease. Heart 2010;96:269-74. [Crossref] [PubMed]


