Uniportal video-assisted thoracoscopic lobectomy
Introduction
Video-assisted thoracic surgery (VATS) anatomic lobectomy for lung cancer was initially described two decades ago (1). Since then, many units have successfully adopted this technique, albeit its precise definition and description greatly vary between them (2).
The final step in the evolution of the technique is the use of a single-port approach. The uniportal access was described initially by Rocco and colleagues for minor thoracic and pulmonary procedures (3). The development of articulated staplers and purposely-designed instruments has helped to perform major pulmonary resections through a single incision approach. The first uniportal VATS lobectomy was described by Gonzalez-Rivas and colleagues, from Coruña University Hospital in 2010 (4). This chapter describes the technique for VATS single-port lobectomies.
Material
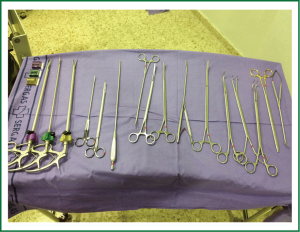
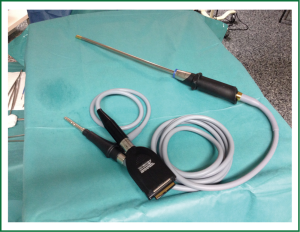
Although uniportal VATS lobectomy can be performed with conventional instruments, the use of especially adapted conventional material (such as instrumentation with both proximal and distal articulation, Figure 1), modern articulated staplers, vascular clips, high definition 30° cameras (The use of videolaparoscope with the distally mounted CCD design facilitates the instrumentation, Figure 2) and energy devices seems to be more fitted for successful single-incision lobectomy.
General aspects
The surgeon and the assistant must to be positioned in front of the patient in order to have the same thoracoscopic vision during all steps of the procedure and experience more coordinated movements (Figure 3).
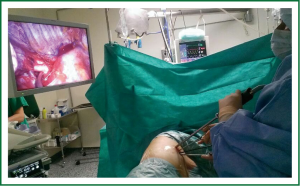
Even though the field of vision is only obtained through the anterior access site, the combined movements of the thoracoscope along the incision will create different angles of vision (in this context, a 30 degree thoracoscope is recommended to achieve a panoramic view). The advantage of using the thoracoscope in coordination with the instruments is that the vision is directed to the target tissue, bringing the instruments to address the target lesion from a direct, sagittal perspective.
Instruments must preferably be long and curved to allow the insertion of 3 or 4 instruments simultaneously (Figure 4). Optimal exposure of the lung is vital in order to facilitate the dissection of the structures and to avoid instrument interference.
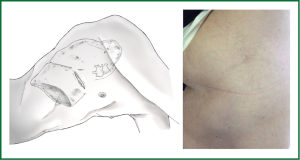
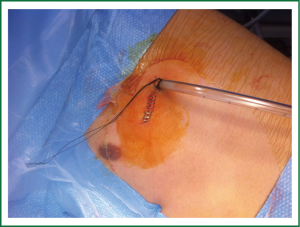
Under general anesthesia and double lumen intubation, the patient is placed in a lateral decubitus position as usual for a conventional VATS. The incision, about 4-5 cm long, is performed preferably in the 5th intercostal space in the anterior position (Figure 5). This location of the incision provides better angles for hilar dissection and insertion of staplers (Video 1). This incision is the same size as the utility incision we use for double or triple port VATS technique to allow removal of specimen (Video 2). There is no need to use a trocar for the thoracoscope.
It is helpful to rotate the surgical table away from surgeons during the hilar dissection and division of structures, and towards the surgeons for the lymph node dissection.
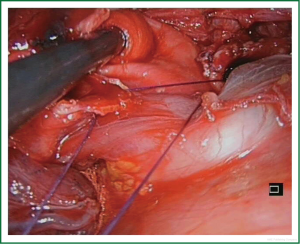
The vessels are usually divided by staplers but when the angle for vascular division is difficult for stapler insertion, the use of vascular clips (click aV, Grena®) or sutures is recommended (Video 3).
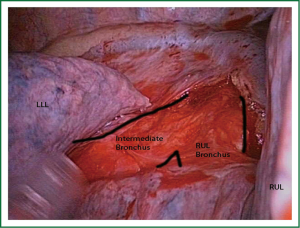
For most of the surgical steps the thoracoscope is usually placed at the posterior part of the utility incision working with the instruments in the anterior part.
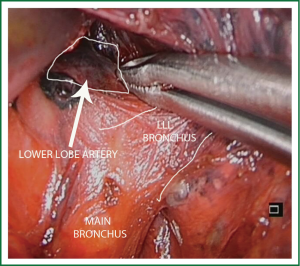
For lower lobectomies the normal sequence of dissection is as follows: inferior pulmonary ligament, inferior pulmonary vein, pulmonary artery, bronchus and finally completion of the fissure. In case of upper lobectomies, the pulmonary artery is normally divided first, followed by vein, bronchus and fissure.
When the lobectomy is completed, the lobe is removed in a protective bag and a systematic lymph node dissection is accomplished. The intercostal spaces are infiltrated with bupivacaine at the end of the surgery under thoracoscopic view. A single-chest tube is placed in the posterior part of the incision (Figure 6). We do not routinely employ epidural or paravertebral catheters.
Indications and contraindications
The indications and contraindication for uniportal VATS are similar as proposed by authors with experience in double or triple-port technique VATS (5).
The only absolute contraindications we consider are surgeon discomfort and huge tumors are not possible to be removed without rib spreading.
Surgical technique
Left lower lobectomy
The lobectomy may be technically different depending on whether the fissure is complete or not. If fissure is complete we try to dissect and staple the artery in the fissure. Sometimes, it is easier to individually divide the arterial branches of the superior and basilar segments. Upon retracting the lobe with a long curved grasper, we cut the pulmonary ligament to find the vein. The vein is dissected free and divided. Then, the lower lobe bronchus is exposed, dissected and stapled the same way as mentioned for the vein. The last step is to staple the fissure and remove the lobe in to a protective bag (Video 4).
In the presence on an incomplete fissure or no visible artery, the technique may change. The preferred method does not involve dissection within the fissure in order to avoid postoperative air leaks. In this case, the lobectomy must be performed from caudal to cranial leaving the fissure stapling as the last step (fissureless technique). Once the lobe is retracted cranially, the sequence of the dissection should be as follows: inferior pulmonary ligament; inferior vein; inferior bronchus. Subsequently, a plane is created between the bronchus and the artery; the artery is taken thus leaving the fissure to be developed last (Video 5).
Right lower lobectomies
The strategy for right lower lobectomy is similar as for the left side but when the artery is exposed in the fissure, the origin of superior segmental artery must to be dissected in the posterior and superior portion of the fissure. The sequence is similar to left lower lobectomy (Video 6).
For lobectomies when performed along a caudo-cranial axis, care must be taken to identify and avoid the damage of the bronchus or artery of the middle lobe. Once the inferior pulmonary vein has been stapled, the lower lobe bronchus is exposed, dissected and divided from its inferior aspect to its bifurcation with the middle lobe bronchus. Dissection of the bronchus with development of the plane between the bronchus and artery is performed leading to the visualization of the artery. We recommend removal of the interbronchial lymph nodes to better define the anatomy. Once identified, the segmental arterial branches to the lower lobe (basilar artery and superior segmental artery) are divided leaving the fissure to be finally stapled (Video 7).
Left upper lobectomy
The operative sequence for left upper lobectomies is similar to conventional VATS. However, we recommend, when feasible, to divide the upper lobe truncus anterior first in order to facilitate division of the upper lobe vein (Figure 7). As a rule, for upper lobes we try to use staplers for all hilar structures. However, when there is no angle for stapler insertion or is difficult from the incision, we either use clips for vascular control (click aV, GrenaR) (Figure 8) or doubly ligates the vessels. We recommend to staple the fissure as the last step of the procedure, after dividing the hilar structures, from anterior to posterior (fissureless technique) (Video 8).
The uniportal view facilitates the dissection and division of upper anterior and apical segmental trunks which are usually hidden by the superior vein when we use a conventional thoracoscopic view. We recommend to first divide the upper anterior and apical segmental trunk in order to facilitate the insertion of the endostaplers in the upper lobe vein. Once this arterial branch is stapled, the vein is easily exposed (Figure 9). It is important to dissect the vein as distal as possible for optimal stapler insertion. The use of curved-tip stapler technology facilitates improved placement around superior pulmonary vein and bronchus through a single incision (Video 9). Another interesting option for management of the upper lobe vein is to open the fissure as the first step, from a hilar view and then dissect the plane between upper and lower vein, with identification of the bronchus and artery. The stapler is inserted over the artery, thereby dividing the fissure, and the lobe is mobilized to allow stapling of the vein from a different angle (Figure 10).
The management of bronchus during left upper lobectomies is more difficult because care must to be taken with lingular artery which lies usually behind the bronchus. We have 4 different forms to manage the upper lobe bronchus (Video 10). The first option consists of exposing the lingular artery and subsequently dividing it in the fissure. At this point, the insertion of an endostapler for the bronchus is easy. In the second option a TA stapler is used for division of the left upper lobe bronchus in certain cases of incomplete fissure to avoid injury of the lingular artery (Figure 11). The third option entails dividing the bronchus with scissors and closing it at the end of the surgery (by manual suture or by using a stapler). The final and fourth option focuses on inserting an endostapler after division of superior trunk (and optionally posterior ascending artery) and vein. This last option must be pursued only by experienced uniportal VATS surgeons.
Right upper lobectomy
The surgical steps are similar to left upper lobectomy: anterior and apical segmental trunk, upper vein, posterior segmental artery, upper bronchus, fissure. We prefer to divide first the upper apico-anterior arterial trunk when possible (Figure 12) to help the insertion of staplers to the upper vein as described in the left upper lobe (Figure 13).
Sometimes it is helpful to partially divide the minor fissure as the first step (anvil of the stapler placed between the upper and middle lobe vein pulling the parenchyma into the jaws of the stapler) in order to get a better angle for the insertion the staplers to the upper vein. This maneuver will provide us with a much better field of vision to dissect and transect the RUL bronchus (Figure 14) or the ascending arteries.
The last step would be to complete the fissure (anvil of the stapler placed over the artery). After transecting the vein, artery and bronchus and after identifying the artery for the middle lobe, we can continue to divide the fissure by placing the stapler over the interlobar artery, pulling the parenchyma anteriorly making sure that the middle lobe artery is left out to the left side of the stapler. The vascular and bronchial stumps are kept out from the staplers jaws (Figure 15).
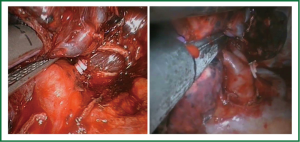
Occasionally, it is better to divide the bronchus after the division of the Boyden trunk to facilitate stapling of the upper lobe vein. We recommend first to expose the posterior bifurcation between the upper lobe and intermediate bronchus by dividing the posterior pleural reflection (Figure 16). This maneuver facilitates the following anterior bronchial dissection and subsequent insertion of staplers to divide the vein.
The use of vascular clips or tie off the vessels is helpful in the division of segmental branches of the pulmonary artery and vein. Except for the management of the bronchus, all vascular branches could be divided using clips rather than staplers.
Middle lobectomy
We recommend to perform the middle lobectomy from caudal to cranial: anterior portion of major fissure, vein, bronchus, artery, anterior portion of minor fissure and finally the posterior portion of fissure. The identification of medium (MLV) and lower lobe vein (LLV) indicates the location to place the stapler to divide the anterior portion of major fissure (the anvil of the stapler is placed between the MLV and LLV, and we pull the parenchyma into the jaws of the stapler). This maneuvre facilitates the dissection and insertion of stapler to transect the vein.
Once the vein is divided, the middle lobe bronchus is exposed, dissected and stapled. A ring forceps is then placed to exert traction onto the middle lobe, thereby exposing the middle lobe artery (medial segmental artery), which is then divided. Finally, the fissures are stapled (Video 11).
Lymphadenectomy
A complete lymph node dissection can be performed with similar results as conventional VATS. For paratracheal dissection it is very helpful the anti-trendelenburg position because it naturally makes the lung “fall down”. For subcarinal dissection the trendelenburg position and the anterior table rotation facilitate the exposure. Preliminary division of the pulmonary ligament gives us a better access to the subcarinal space.
The best location is to place the camera in the upper part of the incision. We can insert 3 or 4 instruments below the camera to complete the systematic dissection of the subcarinal space and paratracheal on the right side and subcarinal and aortopulmonary window on the left side (Video 12).
For left subcarinal dissection, it is helpful to insert two 10 mm endopeanuts in the lower part of the utility incision to retract the aorta, esophagus and lung (Figure 17). This operation facilitates the dissection with instruments placed above the peanuts and below the camera (Video 13).
For right subcarinal lymph node dissection, the esophagus and the intermediate bronchus must be separated to facilitate the procedure (Video 14).
For paratracheal lymph node dissection, we recommend to carry out the procedure by opening the pleura inferiorly to the azygos vein, lifting the azygos vein and retracting the superior vena cava to the right side with an endopath instrument (Figure 18). This technique will create a plane that will allow us to successfully dissect the paratracheal space from an inferior approach (Video 15).
For hilar and N1 station lymphadenectomy, it is important to move and rotate the operating table posteriorly in order to place the lung in the back position. Bimanual instrumentation is crucial to achieve an accurate N1 radical lymph node dissection (Video 16).
Results
Since June 2010, we have performed 222 uniportal VATS major pulmonary resections. All cases were performed by surgeons with experience in VATS surgery, especially in double-port technique for major pulmonary resections and single-port technique for minor procedures (wedge resections, pneumothorax, etc.). This series of patients included advanced NSCLC and complex major resections after chemo-radiotherapy induction treatment.
The overall conversion rate was 3.6%. The most frequent resection was right upper lobectomy (29.4%). The mean surgical time was 151.7±76 minutes (range, 60-310 minutes). After anatomical resection, a complete mediastinal lymphadenectomy was performed in patients with diagnosis of malignancy according to the oncological criteria already adopted in open surgery. The mean number of nodal stations explored was 4.4±1 (range, 3 to 7) with a mean of 14.6±6 (range, 5 to 38) lymph node resections. The mean tumor size was 3±2 cm (range, 0 to 9.8 cm).
The median chest tube duration was 2 days (range, 1 to 16 days) and the median length of stay was 3 days (range, 1 to 58 days).
Discussion
The VATS approach to lobectomy is not standardized. Although 3 to 4 incisions are usually made, the operation can be successfully carried out using only one incision.
Single-port pulmonary resections were initially described by Rocco and colleagues in 2004 (4). Since then they have published different articles on the single-port VATS technique (6,7) for diagnostic and therapeutic procedures, though not including lobectomies. We have adopted this single-incision technique and performed the initial major pulmonary resections by this approach (8,9). Currently we apply the single-port technique for most major resections including complex cases and advanced tumors (10).
The size of the utility incision performed is comparable to the ones commonly used for double (11) or triple-port approach (12). It is essential to accompany the movement of the camera in coordination with the surgical instruments. The use of high definition 30° thoracoscope with the distally mounted CCD design facilitates the instrumentation. The single-port technique provides a direct view to the target tissue. In comparison we feel that the conventional three-port creates an optical plane which requires a torsional angle that is not favorable with standard two-dimension monitors. The parallel instrumentation achieved during the single port approach mimics inside the maneuvers performed during open surgery. The uniportal view facilitates the dissection and division of the upper arterial trunk which is usually hidden by the superior vein when we use a conventional thoracoscopic view. For upper lobectomy, we recommend first dividing the upper lobe truncus anterior to facilitate dividing the upper lobe vein with an endo-stapler. Once the arterial branch is divided the division of the vein is much easier using the single-port approach. It is important to dissect the vein as distal as possible to optimise the passage of the stapler.
An advantage we have noticed is that we don’t need the camera trocar to introduce the lens. By separating the soft tissue we can introduce the camera without trocar allowing us to have more flexibility and obtaining bigger and better angles of vision. Furthermore, we believe that by avoiding the use of the trocar the possibility of an intercostal nerve injury could be minimized. During the instrumentation we always try to avoid putting pressure over the intercostal bundle, putting it over the upper edge of the lower rib to avoid any contact with the nerve. We have the impression that our patients refer less pain when using this approach but we will try to demonstrate it in a further research with a larger patient population. Some authors have reported less postoperative pain and fewer paresthesias in patients operated for pneumothorax through a single incision, in comparison to the classical multiport approach (13).
Single-incision VATS is not only indicated to initial stages or easy cases. With gained experience the most complex cases can be performed in the same manner as with double or triple port approach (14). The previous experience in VATS is important to perform these advanced cases with success. We have performed lobectomies with strong adherences, re-VATS after thoracotomy (Figure 19), tumors with chest wall involvement (Figure 20) (15), cases after induction or radical chemo-radiotherapy, sleeve lobectomies (Figure 21) (16), vascular reconstruction (Figure 22), pancoast tumors, and huge tumors (Figure 23).
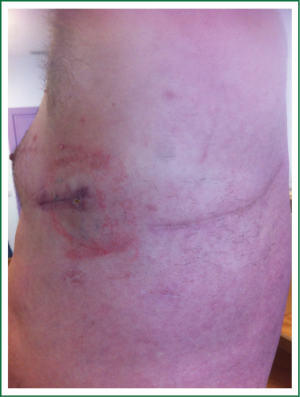
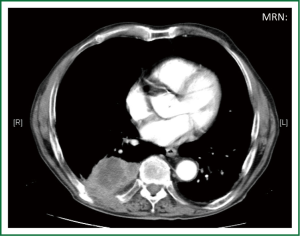
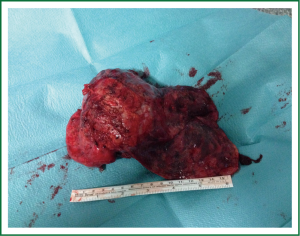
Recently, we have analysed our results comparing early stages of NSCLC with advanced tumors (>5 cm, T3-T4 or after induction therapy) operated by uniportal VATS (87 early stage tumors vs. 47 advanced tumors). Surgical time and number of lymph nodes were higher in advanced tumor group but postoperative outcomes were similar in both groups (chest tube duration, hospital stay and complications). Further analysis of survival for uniportal VATS lobectomy of advanced stage tumors is ongoing.
Despite the increasing adoption of the uniportal VATS approach worldwide, the technique for major lung resections should be learned by implementing dedicated educational pathways inclusive of wet labs and hands on courses as well as visiting experienced VATS centers. We expect further development of new technologies like electrosealing devices for all pulmonary structures, robotic arms that open inside the thorax and wireless cameras, which may allow the uniportal approach to become a standardized addition to the thoracic surgical armamentarium for major pulmonary resections in most thoracic departments.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244-7. [PubMed]
- Rocco G, Internullo E, Cassivi SD, et al. The variability of practice in minimally invasive thoracic surgery for pulmonary resections. Thorac Surg Clin 2008;18:235-47. [PubMed]
- Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg 2004;77:726-8. [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [PubMed]
- McKenna RJ Jr, Mahtabifard A, Swanson SJ. eds. Atlas of minimally invasive thoracic surgery. Saunders 2011;63-5.
- Rocco G, Romano V, Accardo R, et al. Awake single-access (uniportal) video-assisted thoracoscopic surgery for peripheral pulmonary nodules in a complete ambulatory setting. Ann Thorac Surg 2010;89:1625-7. [PubMed]
- Rocco G. One-port (uniportal) video-assisted thoracic surgical resections-A clear advance. J Thorac Cardiovasc Surg 2012;144:S27-31. [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [PubMed]
- Gonzalez-Rivas D, Paradela M, Fieira E, et al. Single-incision video-assisted thoracoscopic lobectomy: Initial results. J Thorac Cardiovasc Surg 2012;143:745-7. [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal Video-Assisted Thoracoscopic Lobectomy: Two Years of Experience. Ann Thorac Surg 2013;95:426-32. [PubMed]
- Burfeind WR, D’Amico TA. Thoracoscopic lobectomy. Op Tech Thorac Cardiovasc Surg 2004;9:98-114.
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5. [PubMed]
- Salati M, Brunelli A, Xiume F, et al. Uniportal video-assisted thoracic surgery for primary spontaneous pneumothorax: clinical and economic análisis in comparison to the traditional approach. Interact Cardiovasc Thorac Surg 2008;7:63-6. [PubMed]
- Hennon M, Sahai RK, Yendamuri S, et al. Safety of thoracoscopic lobectomy in locally advanced lung cancer. Ann Surg Oncol 2011;18:3732-6. [PubMed]
- Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Single-incision thoracoscopic right upper lobectomy with chest wall resection by posterior approach. Innovations (Philadelphia, Pa.) 2013;8:70-2. [PubMed]
- Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Uniportal video-assisted thoracoscopic bronchial sleeve lobectomy: First report. J Thorac Cardiovasc Surg 2013;145:1676-7. [PubMed]