Whole lung lavage—technical details, challenges and management of complications
Whole lung lavage (WLL), technical challenges and management of complications
History
In 1953, Benjamin Castleman encountered a patient with Periodic Acid Schiff stain (PAS) positive proteinaceous material filling the alveoli at the Massachusetts General Hospital. Over the next 5 years, three centers accumulated 27 cases with similar histopathological findings. This series was reported in the New England Journal as a new disease called pulmonary alveolar proteinosis (PAP) (1). As the number of cases recognized increased, the disease was found to be progressive and fatal. Various empirical therapies were proposed. These included antibiotics, corticosteroids and attempts at physical dissolution through administration of potassium iodide, streptokinase, trypsin, heparin and acetylcysteine (2,3). In 1963, Dr. Jose Ramirez-Rivera at the Veterans’ Administration Hospital in Baltimore tried repeated instillation of normal saline by a transtracheal plastic catheter positioned in one lung at a time in a series of two patients. Aliquots of 100 mL of warmed saline were instilled at a rate of 50–60 drops per minute. This process was repeated four times a day for 2–3 weeks. This technique showed improvement in chest-X-ray, diffusion capacity and histo-pathological findings (4,5). It was a prolonged and distressing procedure. The technique was thought to be imperfect and therefore denounced by many physicians at that time (6). In 1964, Ramirez-Rivera used a double lumen endotracheal tube (DLT) to isolate each lung, instilling up to 3 L saline containing heparin or acetylcysteine (7). This trial provided evidence that such a procedure was safe and feasible. Over the next four decades, the procedure has been further refined using general anesthesia, increased lavage volumes (8,9), use of saline alone (10-12) and by performing bilateral sequential WLL in the same treatment session (13).
Indications for WLL
WLL remains the gold standard of therapy for PAP as it provides long lasting benefits in the majority of patients (14).
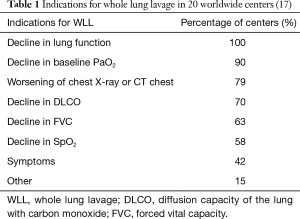
The main indication for WLL is limitation in daily activities due to dyspnea (13,15). Some authors have suggested that patients with PaO2 of less than 70 mmHg on room air or an alveolar-arterial [A-a] oxygen gradient of more than 40 mmHg are more likely to progress and hence require WLL (13,16). Current indications vary from one center to another. In one survey of 20 worldwide centers that perform WLL in adults, the most common indications were declining lung function, declining oxygenation and radiographic worsening (17) (Table 1).
Timing of WLL
In a series of 92 cases, the median time from diagnosis of PAP to performing WLL was 2 months; however some patients did not require therapy for up to 17 years. The majority of patients (79%) underwent WLL within 12 months of diagnosis (5). In another study of 33 patients who underwent WLL, the median period between diagnosis and WLL was 7 months (range 0–60 months) (15).
Interval between treatment of right and left lungs
Although sequential bilateral WLL has been performed safely (14,18), most centers perform WLL in separate sessions for each lung. The time between WLL for each lung is around 3 weeks (17). Patients usually have improved symptoms and oxygenation by 3 weeks making the second lavage safer.
Management of anticoagulation prior to WLL
There is no specific data about management of anti-coagulation or anti platelets therapy prior to WLL. We recommend that bronchoscopy and BAL guidelines be used as a guide for WLL (19). We recommend a platelet count of >50,000, international normalized ratio (INR) of <1.5. Full anticoagulation should be stopped for the procedure. The decision to bridge with heparin depends on the risk of thromboembolic event as appropriate by ACCP guidelines. The procedure should be delayed if the patient has had a bare metal stent or myocardial infarction in the last 6 weeks, or a drug eluting stent in the last 6 months, necessitating the use of clopidogrel or newer antiplatelet agents (20). Aspirin does not need to be held (20). It is worth mentioning that extracorporeal membrane oxygenation (ECMO) with anticoagulation has been used for severe cases of PAP, without any report of bleeding (16,21-24).
Technical aspects of WLL
Due to the rarity of the disease and the lack of randomized trials, there are no specific guidelines for the technique of performing WLL. Each institution has its own protocol with slight variations. The technique discussed in the following section and in Table 2 is practiced at the university of Oklahoma Health Sciences Center.

Full table
Personnel
An experienced lavage team that includes nursing, anesthesiology, respiratory therapy and interventional pulmonary medicine is essential to perform this procedure safely and effectively (25).
Anesthesia for WLL
WLL is performed under general anesthesia, usually with a combination of intravenous propofol, opioid and a neuromuscular blocker (17). Because of its bronchodilation effect, inhalational anesthesia has been used in patients with previous history of asthma or bronchospasm (25).
Parameters typically monitored during anesthesia include electrocardiogram, arterial oxygen saturation, noninvasive blood pressure, invasive blood pressure with an arterial catheter, end tidal CO2, arterial blood gas analysis and a bispectral index which is used to monitor the depth of the anesthesia (17).
Isolation of the lung
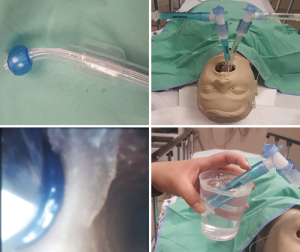
Following induction of general anesthesia, a left sided DLT is introduced by an experienced anesthesiologist. A left DLT is preferred over a right sided DLT primarily to avoid blocking the takeoff of the right upper lobe bronchus (26). A flexible pediatric bronchoscope is used to confirm the placement of DLT and the inflation of the cuffs. Isolation of each lung is confirmed by ventilating one lung, and checking for air leak by venting the non-ventilated lung endotracheal tube orifice into saline water seal cup while the ventilated lung is held at an airway pressure of 40–50 cm water pressure. The absence of air bubbles confirms appropriate isolation of both lungs (Figure 1).
Body position
Multiple body positions were described during WLL, with a variety of rationales. Supine (18,25,26), prone (25), lateral decubitus positioning with lavaged lung up (14), lateral decubitus positioning with lavaged lung down and reverse Trendelenburg positions have been used (17,18).
Prone, lateral decubitus positioning with lavaged lung up and reverse Trendelenburg positions have been reported to result in better drainage of the fluid (17,18).
Lateral decubitus positioning with lavaged lung up has also been described to decrease ventilation perfusion mismatching and hypoxia during WLL by decreasing perfusion to the lavaged lung while increasing the perfusion of the dependent ventilated lung (14,17). Lateral decubitus position with the lavaged lung down has been described to help prevent spillage into the contralateral lung (17).
The evidence for the benefit from a particular body position during lavage is limited. All of these positions increase the risk of dislodging the double lumen tube. Repositioning the tube is difficult during the lung lavage due to rapid spillage of the volume instilled and frothing which obscures visualization, even with bronchoscopy. Overall, there is little data to routinely recommend any one position other than supine for WLL.
Lung selection
The most severely affected lung should be lavaged first. Earlier studies reported the use of Broncho-spirometry. The oxygen uptake of each lung is determined separately and the most severely affected side is lavaged initially (25). This approach has been abandoned. In one survey, 12/20 centers relied on radiographic information only to treat the more severely affected lung initially and in 6/20 centers the left lung was always treated first (17). If imaging is equivocal, we usually obtain a perfusion scan and aim to treat the less perfused side first. By ventilating the lung with the better perfusion and optimizing the ventilation and perfusion ratio, single lung ventilation can be tolerated better.
Degassing and filling of the lung
In preparation for the procedure, 3 L bags of normal saline are warmed to 37 °C in warming basins (ECOLAB ORS 2000 series irrigation solution warmer, Saint Paul, Minnesota, USA) (Figure 2). Alternatively, blood transfusion warmers can be used to warm the saline (27). A total saline volume of 30–50 Liters should be anticipated.
A closed system (Figures 2,3) is created by using a Y connector (we use a high flow irrigating Y-set adapter from smith medical) with tubing that is connected to:
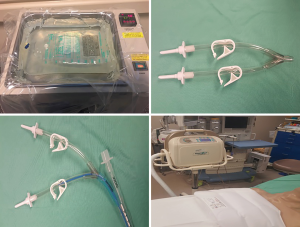
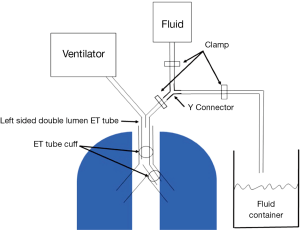
- the ET tube lumen ending in the targeted lung;
- the normal saline bags;
- a drainage container.
We start de-nitrogenating the lung by ventilating with 100% oxygen for 15–20 minutes (the longer duration of treatment is to compensate for the heterogeneous ventilation in the diseased lung). The remaining oxygen in the target lung following denitrogenation is expected to be consumed at an approximate rate of 125 mL/min (assuming a basal oxygen consumption of 250 mL/min) (28). To prevent barotrauma and bubble formation, the initial aliquot is infused no faster than this rate. Alternatively, some centers achieve degassing of one lung by ventilation with 100% oxygen followed by forced deflation of the lung with negative airway pressure and subsequent airway occlusion that is maintained for 10–15 min up to absorption atelectasis of the whole lung (17).
The volume to be infused in the first aliquot is estimated as half of the FRC measured at full body plethysmography before the procedure. The saline is infused under gravity with the bags hanging 50 cm above the mid-axillary line. This sets the maximal hydrostatic pressure that drives the saline into the lung and is the pressure at which the seal of the double lumen tube is confirmed to avoid contamination of the other lung with lavage fluid.
Warm normal saline is introduced into the target lung through this closed system until the estimated FRC value has been reached. At this point, the lung is completely de-gassed and partially filled with saline.
An alternative technique for WLL entails instilling 1L aliquots of saline and draining it without filling the lung up to FRC. Before starting, the lung to be treated is left unventilated for 5 minutes to allow degassing (in this case the absorption of sufficient oxygen to allow for the instillation of the 1 L of saline) (27).
Drainage and instillation cycle
Incremental tidal volumes of 500–1,000 mL of saline are allowed to flow into the lung under the gravitational force of the saline column. This force does not exceed the measured height of the saline column of 50 cm. The effluent is allowed to drain via the closed system into a measuring cylinder.
Chest percussion with a wraparound vest is applied for 3–5 minutes, both at maximal volume and during drainage in each cycle (Figure 2). The saline is drained to gravity or with active suction back down to FRC. The initial returns are typically milky or turbid. Each cycle takes 3–5 minutes. Cycles can take longer if the patient has concomitant asthma. The process of filling and washing the lungs is continued until the effluent becomes clear. Then the remaining fluid can be drained completely while percussion is being done.
Manual percussion of the chest wall by a physiotherapist can be performed in order to increase clearance of proteinaceous material. Percussion can be administered during both instillation and removal of fluid .The role of manual percussion versus hand-held mechanical percussion device has been investigated. Hammon et al. demonstrated that optical density of recovered lavage fluid was greater from patients who received manual percussion than those who received percussion using hand held mechanical devices or no percussion (29). However, manual percussion results in significant soreness after the procedure. Mechanical percussion with a vest causes less discomfort after the procedure, is better tolerated and is less labor intensive than manual percussion.
Intraoperative monitoring
Intraoperative monitoring of the patient’s level of oxygenation is accomplished by following oxygen saturation as well as serial readings of arterial blood gas levels. The physiologic alterations during the lavage can be anticipated from the hemodynamic response of the pulmonary circulation to the variations in the airway pressure that occur as the airways are cyclically filled and emptied. The highest oxygen saturation is usually seen at the completion of the filling phase when the blood is physiologically shunted from the non-ventilated to the ventilated lung. Conversely the oxygen level will drop as the lavage lung is emptied (25).
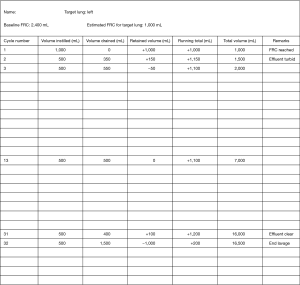
The volume of saline infused and recovered should be closely monitored (Figure 4). Large losses of fluid can indicate leakage into the contralateral lung or the pleural space. Leakage into the contralateral lung is usually recognized by observing lavage fluid in the endotracheal tube of the ventilated lung. This can be fixed by adjustment of the bronchial balloon cuff pressure or by repositioning the double lumen ET tube. Bronchoscopic examination to confirm and adjust the position of the ET tube may be needed. Leakage into the ipsilateral pleural space can be detected by performing a bedside ultrasound examination.
The total volumes of saline required can be up to 40 L. In a survey of 20 centers performing WLL, the average total volume used was 15.4±6.8 L per lung (17). Adjusted for body weight, a total volume of 250 mL/kg can be used on average in adults and children (30).
Post-procedure care
At the termination of the procedure, the residual saline is drained and aspirated from the lung and ventilation with 100% oxygen is resumed. The DLT is then replaced with a single lumen endotracheal tube. Bronchoscopy can be performed at this time to aspirate any residual fluid. Diuretics can be used to help in clearing the fluid from the lung (14). The patient is monitored in the recovery unit for an hour on mechanical ventilation. At the end of the recovery time the patient is awakened and extubated. The patient is further monitored and can be discharged home on the same day.
Variations in technique
Single vs. double lung lavage
In contrast to our practice, bilateral sequential lung lavage is done routinely during the same procedure in some centers (14,17). This is done with the patient supine. The more severely affected lung is lavaged first and 1L aliquots are used as described previously with isolation of the lung achieved using a DLT. When the effluent is clear from one lung, it is drained completely. Then dual lung ventilation is started. Positive end expiratory pressure (PEEP) and diuretics are used to help re-inflate the lavaged lung. This can take up to an hour. Once the patient is able to tolerate single lung ventilation on the lavaged lung, the other lung is lavaged using the same 1 L aliquots as described. After the procedure is completed, the DLT is switched to a single lumen endotracheal tube. In contrast to the single lung lavage procedure, these patients frequently require overnight mechanical ventilation. Most are extubated in 18 hours after the termination of the procedure. The procedure itself takes around 8 hours (14).
Modified WLL
A modification of the lung lavage technique has been described by Bingisser et al., in a case where routine lavage was not effective, the lung was partially drained after filling with saline and then ventilated with PEEP (5-10 cm H2O). This was designed to increase clearance of proteinaceous material. Some centers use this procedure routinely. It is associated with an increased risk of barotrauma and resulting hydro-pneumothorax (31).
ECMO
Patients with respiratory failure related to advanced PAP (16,21-24) with or without pulmonary hypertension related to long standing PAP as well as patients with previous lung resections, might be considered for ECMO while performing WLL. These patients are not able to tolerate single lung ventilation. Although strict criteria are not required, it has been suggested that ECMO may be considered when the PaO2 is less than 100 mmHg on an FIO2 of 1 (25).
Complications of WLL
In a survey by Campo et al. that includes 30 centers, an estimated 1,110 WLL procedure were performed over a period of 18±11 years for an average of 5.6±5 procedure per center per year. The most common reported complications were fever, which occurred in 18% followed by hypoxemia (14%), wheezing (6%), pneumonia (5%) and fluid leakage (4%). Pleural effusion and pneumothorax occurred in 3.1% and 0.8% respectively. No patient needed prolonged mechanical ventilation (17). Cardiac arrest was reported to occur in one case (17). One case of death related to WLL has been reported as well (32). It is likely that mortality is under reported.
The major side effect from WLL is hypoxemia which can be improved by ventilation with high inspired oxygen concentration (33,34). As previously discussed, during the filling phase, arterial oxygenation improves due to increase in airway pressure and redirection of blood to the contralateral ventilated lung (13,20). Emptying of the lung causes a decrease in airway pressure and perfusion of the lung undergoing treatment resulting in a fall in PaO2 (13). Excessive PEEP in the ventilated lung should be avoided, as this may shunt the blood away to the non-ventilated lung leading to ventilation/perfusion mismatch and worsening oxygenation (35).
Mal-positioning of the double lumen endobronchial tube with spillage of lavage fluid into the ventilated lung can occur (13). Barotrauma related to rapid instillation of large volumes of fluid can result in hydro-pneumothorax, subcutaneous emphysema and pleural fluid collections (33,36). Large pleural effusion at the end of the procedure can be drained with thoracentesis to aid in extubation.
Hypothermia can occur and is minimized by close monitoring of patient’s body temperature, keeping the body warm using heating pads and using saline warmed to body temperature.
Change in lung mechanics and outcomes after WLL
Due to lack of randomized clinical trials, the true therapeutic efficacy of WLL cannot be determined. Based on available data, the 5-year survival rate of patients who underwent WLL was significantly better compared to those who did not receive WLL therapy (94±2% vs. 85±5%) (5).
The timing of repeat WLL is variable and tailored according to the patient’s symptoms, with severe symptoms requiring early repeat procedure (37). In one study, the median symptom free time after first WLL was 3 years, with 70% of patients followed beyond 3 years remaining free of recurrent PAP symptoms (14). In another report a much shorter median duration of benefit of 15 months was reported, after which patients required a repeat lavage (5).
Clinically, WLL has been associated with an improvement in dyspnea (14). There is also an improvement in objective parameters. A report of 47 patients with paired pre-lavage and post-lavage data demonstrate that there was significant improvement in PaO2, [A-a] gradient, FEV1, vital capacity and DLCO (34). More recently, a study assessing the long-term effect of WLL reported an immediate improvement in FVC, with the effect persisting up to 1-year after the procedure (14). The DLCO and the 6-minute walk distance also showed improvement that persisted at 6 months following WLL (14).
Conclusions
WLL remains first line therapy for PAP. Considerable knowledge and experience has been acquired in the performance of this procedure since it was initially described in 1964. It should only be performed in specialized centers using a skilled team that includes anesthesiology, respiratory therapy and pulmonary medicine. Careful planning and execution of this procedure is essential to optimize safety and efficacy.
Acknowledgements
We would like to acknowledge Jamie Stringfellow, RRT and Loan Nguyen, MD for their help in acquiring media images.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rosen SH, Castleman B, Liebow AA, et al. Pulmonary Alveolar Proteinosis. New England Journal of Medicine 1958;258:1123-42. [Crossref] [PubMed]
- Davidson JM, Macleod WM. Pulmonary alveolar proteinosis. Br J Dis Chest 1969;63:13-28. [Crossref] [PubMed]
- De Sanctis PN. Pulmonary alveolar proteinosis. A review of the findings and theories to date, with a digression on Pneumocystis carinii pneumonia. BMQ 1962;13:19-35. [PubMed]
- Ramirez J, Schultz RB, Dutton RE. Pulmonary Alveolar Proteinosis: A New Technique and Rationale for Treatment. Arch Intern Med 1963;112:419-31. [Crossref] [PubMed]
- Seymour JF, Presneill JJ. Pulmonary alveolar proteinosis: progress in the first 44 years. Am J Respir Crit Care Med 2002;166:215-35. [Crossref] [PubMed]
- Ramírez-Rivera J. The strange beginnings of diagnostic and therapeutic bronchoalveolar lavage. P R Health Sci J 1992;11:27-32. [PubMed]
- Ramirez J, Kieffer RF Jr, Ball WC Jr. Bronchopulmonary lavage in man. Ann Intern Med 1965;63:819-28. [Crossref] [PubMed]
- Ramirez J. Pulmonary alveolar proteinosis. Treatment by massive bronchopulmonary lavage. Arch Intern Med 1967;119:147-56. [Crossref] [PubMed]
- Wasserman K, Blank N, Fletcher G. Lung lavage (alveolar washing) in alveolar proteinosis. Am J Med 1968;44:611-7. [Crossref] [PubMed]
- Wasserman K. Evaluation of solutions used for lung lavage in alveolar proteinosis. Rounds of the Teaching Staff of Wadsworth Hospital Centre 1968;11:217-22.
- Kao D, Wasserman K, Costley D, et al. Advances in the treatment of pulmonary alveolar proteinosis. Am Rev Respir Dis 1975;111:361-3. [PubMed]
- Sunderland WA, Klein RL. Heparin absorption during heparin-saline lung lavage in a patient with pulmonary alveolar proteinosis. Chest 1973;63:1033-4. [Crossref] [PubMed]
- Shah PL, Hansell D, Lawson PR, et al. Pulmonary alveolar proteinosis: clinical aspects and current concepts on pathogenesis. Thorax 2000;55:67-77. [Crossref] [PubMed]
- Beccaria M, Luisetti M, Rodi G, et al. Long-term durable benefit after whole lung lavage in pulmonary alveolar proteinosis. Eur Respir J 2004;23:526-31. [Crossref] [PubMed]
- Gay P, Wallaert B, Nowak S, et al. Efficacy of Whole-Lung Lavage in Pulmonary Alveolar Proteinosis: A Multicenter International Study of GELF. Respiration 2017;93:198-206. [Crossref] [PubMed]
- Cooper JD, Duffin J, Glynn MF, et al. Combination of membrane oxygenator support and pulmonary lavage for acute respiratory failure. J Thorac Cardiovasc Surg 1976;71:304-8. [PubMed]
- Campo I, Luisetti M, Griese M, et al. Whole lung lavage therapy for pulmonary alveolar proteinosis: a global survey of current practices and procedures. Orphanet J Rare Dis 2016;11:115. [Crossref] [PubMed]
- Silva A, Moreto A, Pinho C, et al. Bilateral whole lung lavage in pulmonary alveolar proteinosis--a retrospective study. Rev Port Pneumol 2014;20:254-9. [Crossref] [PubMed]
- Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax 2013;68:i1-i44. [Crossref] [PubMed]
- Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: american college of chest physicians evidence-based clinical practice guidelines. Chest 2012;141:e326S-e50S.
- Zapol WM, Wilson R, Hales C, et al. Venovenous bypass with a membrane lung to support bilateral lung lavage. Jama 1984;251:3269-71. [Crossref] [PubMed]
- Sivitanidis E, Tosson R, Wiebalck A, et al. Combination of extracorporeal membrane oxygenation (ECMO) and pulmonary lavage in a patient with pulmonary alveolar proteinosis. Eur J Cardiothorac Surg 1999;15:370-2. [Crossref] [PubMed]
- Freedman AP, Pelias A, Johnston RF, et al. Alveolar proteinosis lung lavage using partial cardiopulmonary bypass. Thorax 1981;36:543-5. [Crossref] [PubMed]
- Cohen ES, Elpern E, Silver MR. Pulmonary alveolar proteinosis causing severe hypoxemic respiratory failure treated with sequential whole-lung lavage utilizing venovenous extracorporeal membrane oxygenation: a case report and review. Chest 2001;120:1024-6. [Crossref] [PubMed]
- Claypool WD, Rogers RM, Matuschak GM. Update on the clinical diagnosis, management, and pathogenesis of pulmonary alveolar proteinosis (phospholipidosis). Chest 1984;85:550-8. [Crossref] [PubMed]
- Abdelmalak BB, Khanna AK, Culver DA, et al. Therapeutic Whole-Lung Lavage for Pulmonary Alveolar Proteinosis: A Procedural Update. Journal of Bronchology & Interventional Pulmonology 2015;22:251-8. [Crossref] [PubMed]
- Michaud G, Reddy C, Ernst A. Whole-lung lavage for pulmonary alveolar proteinosis. Chest 2009;136:1678-81. [Crossref] [PubMed]
- Kwan M, Woo J, Kwok T. The standard oxygen consumption value equivalent to one metabolic equivalent (3.5 ml/min/kg) is not appropriate for elderly people. Int J Food Sci Nutr 2004;55:179-82. [Crossref] [PubMed]
- Hammon WE, McCaffree DR, Cucchiara AJ. A comparison of manual to mechanical chest percussion for clearance of alveolar material in patients with pulmonary alveolar proteinosis (phospholipidosis). Chest 1993;103:1409-12. [Crossref] [PubMed]
- Paschen C, Reiter K, Stanzel F, et al. Therapeutic lung lavages in children and adults. Respiratory Research 2005;6:138. [Crossref] [PubMed]
- Bingisser R, Kaplan V, Zollinger A, et al. Whole-lung lavage in alveolar proteinosis by a modified lavage technique. Chest 1998;113:1718-9. [Crossref] [PubMed]
- Bhagwat AG, Wentworth P, Conen PE. Observations on the relationship of desquamative interstitial pneumonia and pulmonary alveolar proteinosis in childhood: a pathologic and experimental study. Chest 1970;58:326-32. [Crossref] [PubMed]
- Prakash UB, Barham SS, Carpenter HA, et al. Pulmonary alveolar phospholipoproteinosis: experience with 34 cases and a review. Mayo Clin Proc 1987;62:499-518. [Crossref] [PubMed]
- Rogers RM, Levin DC, Gray BA, et al. Physiologic effects of bronchopulmonary lavage in alveolar proteinosis. Am Rev Respir Dis 1978;118:255-64. [PubMed]
- Julien T, Caudine M, Barlet H, et al. Ann Fr Anesth Reanim 1986;5:173-6. [Effect of positive end expiratory pressure on arterial oxygenation during bronchoalveolar lavage for proteinosis]. [Crossref] [PubMed]
- Kariman K, Kylstra JA, Spock A. Pulmonary alveolar proteinosis: prospective clinical experience in 23 patients for 15 years. Lung 1984;162:223-31. [Crossref] [PubMed]
- Borie R, Danel C, Debray MP, et al. Pulmonary alveolar proteinosis. Eur Respir Rev 2011;20:98-107. [Crossref] [PubMed]