Re-visiting D-dimers and fibrin degradation products for the diagnosis of acute aortic dissection
Acute aortic dissection (AAD), the most severe form of acute aortic syndrome, is a catastrophic condition and a challenge to physicians (1). The heterogeneous clinical presentations of AAD may delay a correct diagnosis and proper management, which affects surgical outcomes and long-term consequences (2). The prevalence of AAD in emergency departments was estimated to be 1 in 10,000 from a report in 2012 (3). About 27% AAD patients present with a classical triad of sudden and severe tearing chest pain, an extremity blood pressure differential of >20 mmHg, and a widened mediastinum observed in chest radiographs (3,4). On the other hand, nearly 4% patients with AAD present none of the classical symptoms (4). The presence of non-classical symptoms, thereby a common clinical scenario of AAD, tended to delay diagnosis for longer than 12 hours as compared with the median time of 3 hours (5). A potential for diagnosis delayed for more than 24 hours has been reported in up to 39% patients after hospitalization (4). In addition, over 60% women with AAD are reportedly older than 65 years of age and likely to present atypical symptoms, which leads to delayed diagnosis (1,6). Nonspecific symptoms of the “great masquerader” include angina, abdominal pain, pleural effusion, neurologic symptoms, syncope, dyspnea, and acute limb ischemic pain, and are reported to be commonly present in over 15% patients visiting emergency departments (7). It is of note that acute coronary syndrome was among the most common erroneous diagnoses that led to antithrombotic and antiplatelet therapies (5). These data highlight an unmet medical need in modern medicine for efficient and accurate diagnostic tools to combat AAD.
Imaging studies using computed tomography (CT), magnetic resonance imaging (MRI), or transesophageal echocardiography have shown high sensitivity and specificity (1). However, these imaging tools are invasive and are not the first line screening armamentarium for non-classical AAD symptoms in the emergency departments. Testing for serum biomarkers of AAD provides a reasonable and logical step before proceeding to imaging studies or appropriate therapies. Serum levels of smooth muscle myosin heavy chain and creatine kinases were first shown to be correlated to AAD in the 1990s (Table 1). Since the first analysis for AAD patients in 2003, the serologic D-dimer test was extensively studied in aortic diseases, and this was included as one of the laboratory test items for AAD in the European guidelines on the diagnosis and treatment of aortic diseases (level of evidence IIa, level of evidence B) (14). A diagnosis of AAD should be included when the serum D-dimer levels exceed the threshold of 0.5 µg/mL (or 2.738 nmol/L) (1). Low serum D-dimer levels may exclude AAD, but not the possibilities of intramural hematoma and penetrating aortic ulcer (14).
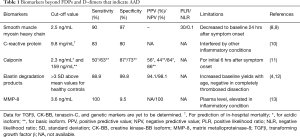
Full table
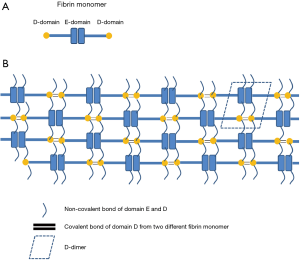
D-dimer is the end-product obtained after a cleavage of the fibrin polymer, which is composed of a conglomeration of fibrin monomers containing a central E-domain with two distal D-domains (Figure 1A) (15). When tissue injury occurs, platelets, in combination with the coagulation cascade, are activated. The soluble protein fibrinogen is thereby converted to an insoluble protein fibrin monomer by thrombin, an enzyme product that is generated from the coagulation cascade. Fibrin monomers then aggregate to form fibrin protofibrils through non-enzymatic associations, which are further consolidated by coagulation factor XIIIa, in order to form a cross-linked fibrin polymer (Figure 1B). Fibrin cleavage is a repeated step of digestion that occurs at specific sites with the help of plasmin, an activated form of plasminogen present in the interstitial spaces of organs including vascular tissue (15). The degradation products are variable in size, or molecular weight, and are generally called fibrin degradation products (FDP). The terminal FDPs are the D-D-E domain fragments, namely D-dimers (Figure 1B). Commercially available kits for D-dimer tests target not only D-D-E fragments but also FDP with D-dimer antigens (16). Under certain pathological conditions, such as disseminated intravascular coagulopathy, fibrinogen is degraded through plasmin before being activated into fibrin monomers. The degradation products include the fragments of D and E-domain molecules, not D-dimers. Commercially available FDP kits detect degradation fragments from both fibrin clots and fibrinogen.
On March 06, 2017, in the issue of Scientific Reports (17), Dong et al. shared the results of a retrospective study to differentiate AAD patients (n=202) from non-AAD and healthy controls by using circulating levels of FDP and D-dimers, which were measured through latex immunoturbidimetric assays. The diagnosis for the non-AAD group included myocardial infarction (n=45), pulmonary infarction (n=51), and abdominal aortic aneurysm (n=54). The significantly low fibrinogen levels in the AAD and non-AAD groups corresponded with the findings of high FDP and D-dimer levels. The area under the curve (AUC) of the receiver operating characteristic (ROC) curve was around 0.66 for both FDP and D-dimer so as to differentiate between AAD and non-AAD patients. The AUC for the ROC curve was around 0.86 for both FDP and D-dimer to differentiate the AAD patients from healthy controls. Dong et al. did not list the cut-off values for such differentiation in the study. Previous meta-analyses suggested a threshold of 0.5 µg/mL using D-dimer, which showed a pooled sensitivity of 98% and specificity of 42% (18,19). However, the positive predictive value is generally under 50% at the 0.5 µg/mL threshold of D-dimer levels due to the low prevalence rate of AAD in the emergency departments (19). Hagiwara et al. proposed a D-dimer plasma level ≥3.8 µg/mL or FDP °√12.6 µg/mL for the consideration of a chest CT with contrast (20). The threshold should be lowered for AAD patients with complete thrombosis in the false lumen (20). In addition, Hagiwara et al. found a strong correlation (R2 =0.97) between D-dimer and FDP data, implicating that D-dimer might be representative of FDP in an AAD population, and vice versa (20). D-dimer, rather than FDP, is commonly used to rule-out AAD in clinical settings.
In spite of the high sensitivity and AUC data in the above mentioned studies, Moysidis T et al. found that the chances of an elevated D-dimer value were lower than 30% among the patients who had chest pain of non-cardiac origin (21). It is generally recommended that D-dimer should be used in conjunction with clinical presentation to diagnose AAD (14,18,21). The American Heart Association provided a risk score that was based on prior medical history, clinical symptoms, and physical examination findings (14,18). The risk score assists in determining the use of D-dimer and imaging studies for the diagnosis of AAD. The diagnostic pathway using systemic D-dimer screening for AAD, without consideration of other clinical information, among patients with chest pain had resulted in a nearly 40% increase of the CT images which were negative for AAD (21). It should also be noted that the baseline D-dimer level is increased with age, and among pregnant cases (15). Nevertheless, all the above-mentioned studies consisted of over 60% male patients, an inevitable gender bias for AAD. Consequently, FDP and D-dimer biomarkers should be used with caution for females and the elderly (>70 years) patients (15). Other potential markers such as smooth muscle myosin, matrix metalloproteinase (14), elastin degradation products, transforming growth factor-β, and tenascin-C were still under investigation and had not been applied clinically (Table 1). Newly explored markers such as microRNA (miR-21-5p) and growth factor genes might also shed some new light on early detection of AAD among the patients with non-classical symptoms (22).
In summary, D-dimer or FDP tests could be considered for those with low probability of AAD, at least within 48 hours after the symptom onset. However, negative test results should not preclude the need for a subsequent imaging survey. It is as yet unclear whether D-dimer or FDP tests can shorten the time taken to diagnose AAD. New diagnostic biomarkers are under investigation, and they may be promising. An efficient way to identify AAD from variable and atypical symptoms is to stay updated with comprehensive knowledge about the disease with a respectful mind.
Acknowledgements
Funding: This work was supported by a grant from the National Science Council of Taiwan (Grant: MOST 105-2314-B-006-068-to JNR).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mussa FF, Horton JD, Moridzadeh R, et al. Acute aortic dissection and intramural hematoma: a systematic review. JAMA 2016;316:754-63. [Crossref] [PubMed]
- Roan JN, Wu HY, Luo CY. Risk factor analysis of surgical and long-term results in patients with acute type A aortic dissection. In: Chen YF, Luo CY, Dominique ST, editors. Recent advances in acute type A aortic dissection. 1st edition. Sharjah, U.A.E.: Bentham Science Publishers Ltd; 2015:265-85.
- Klompas M. Does this patient have an acute thoracic aortic dissection? JAMA 2002;287:2262-72. [Crossref] [PubMed]
- Strayer RJ, Shearer PL, Hermann LK. Screening, evaluation, and early management of acute aortic dissection in the ED. Curr Cardiol Rev 2012;8:152-7. [Crossref] [PubMed]
- Rapezzi C, Longhi S, Graziosi M, et al. Risk factors for diagnostic delay in acute aortic dissection. Am J Cardiol 2008;102:1399-406. [Crossref] [PubMed]
- Nienaber CA, Fattori R, Mehta RH, et al. Gender-related differences in acute aortic dissection. Circulation 2004;109:3014-21. [Crossref] [PubMed]
- Taylor RA, Iyer NS. A decision analysis to determine a testing threshold for computed tomographic angiography and D-dimer in the evaluation of aortic dissection. Am J Emerg Med 2013;31:1047-55. [Crossref] [PubMed]
- Suzuki T, Lyon A, Saggar R, et al. Editor's choice-biomarkers of acute cardiovascular and pulmonary diseases. Eur Heart J Acute Cardiovasc Care 2016;5:416-33. [Crossref] [PubMed]
- Suzuki T, Katoh H, Nagai R. Biochemical diagnosis of aortic dissection: from bench to bedside. Jpn Heart J 1999;40:527-34. [Crossref] [PubMed]
- Vrsalovic M, Zeljkovic I, Presecki AV, et al. C-reactive protein, not cardiac troponin T, improves risk prediction in hypertensives with type A aortic dissection. Blood Press 2015;24:212-16. [Crossref] [PubMed]
- Ranasinghe AM, Bonser RS. Biomarkers in acute aortic dissection and other aortic syndromes. J Am Coll Cardiol 2010;56:1535-41. [Crossref] [PubMed]
- Shinohara T, Suzuki K, Okada M, et al. Soluble elastin fragments in serum are elevated in acute aortic dissection. Arterioscler Thromb Vasc Biol 2003;23:1839-44. [Crossref] [PubMed]
- Giachino F, Loiacono M, Lucchiari M, et al. Rule out of acute aortic dissection with plasma matrix metalloproteinase 8 in the emergency department. Crit Care 2013;17:R33. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Olson JD. D-dimer: an overview of hemostasis and fibrinolysis, assays, and clinical applications. Adv Clin Chem 2015;69:1-46. [Crossref] [PubMed]
- Adam SS, Key NS, Greenberg CS. D-dimer antigen: Current concepts and future prospects. Blood 2009;113:2878-87. [Crossref] [PubMed]
- Dong J, Duan X, Feng R, et al. Diagnostic implication of fibrin degradation products and D-dimer in aortic dissection. Sci Rep 2017;7:43957. [Crossref] [PubMed]
- Asha SE, Miers JW. A systematic review and meta-analysis of D-dimer as a rule-out test for suspected acute aortic dissection. Ann Emerg Med 2015;66:368-78. [Crossref] [PubMed]
- Suzuki T, Distante A, Zizza A, et al. Diagnosis of acute aortic dissection by D-dimer: the International Registry of Acute Aortic Dissection Substudy on Biomarkers (IRAD-Bio) experience. Circulation 2009;119:2702-07. [Crossref] [PubMed]
- Hagiwara A, Shimbo T, Kimira A, et al. Using fibrin degradation products level to facilitate diagnostic evaluation of potential acute aortic dissection. J Thromb Thrombolysis 2013;35:15-22. [Crossref] [PubMed]
- Moysidis T, Lohmann M, Lutkewitz S, et al. Cost associated with D-dimer screening for acute aortic dissection. Adv Ther 2011;28:1038-44. [Crossref] [PubMed]
- Kimura N, Futamura K, Arakawa M, et al. Gene expression profiling of acute type A aortic dissection combined with in vitroassessmentdagger. Eur J Cardiothorac Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]


