Decreased Wnt4 expression inhibits thymoma development through downregulation of FoxN1
Introduction
The thymus provides a specialized microenvironment for the development and selection of mature T cells. The development of thymic epithelial cells (TECs) is regulated by multiple signaling levels, and the transcription factor forkhead box N1 (FoxN1) is the key regulator of this process (1). FoxN1 expression is strongly regulated by the wingless (Wnt) signaling pathway and bone morphogenetic protein (BMP) signaling in TECs (2,3). Inhibition of the Wnt signaling pathway in TECs is a factor that initiates thymic degeneration via the loss of FoxN1 expression (4). The retroviral transfection of TEC lines to induce overexpression of Wnt4 and Wnt5b was shown to significantly increase Foxn1 expression, while the specific blocking of Wnt4 protein by Frizzled (Fz) receptors or of endogenous Wnt signaling decreased FoxN1 expression (5). Recently, Kvell et al. (6) discovered that Wnt4 is capable of increasing the expression level of characteristic intracellular (FoxN1), surface (MHCII), and secreted (IL17) molecules, and when Wnt/beta-catenin was inhibited by inhibitor of beta-catenin and TCF-4 (ICAT), the expression of these molecules was moderately decreased.
Thymomas are rare tumors arising from TECs; they most commonly arise in the anterosuperior mediastinum, with an incidence rate of approximately 2.5 per million per year (7). The pathogenesis of thymoma is poorly understood given the complexity of its pathological features and the lack of basic research. Our preliminary study showed activation of the Wnt4 signaling pathway and the abnormal expression of FoxN1 in thymoma (8). However, further study is required to prove whether the regulatory relationship between the Wnt signaling pathway and FoxN1 in thymoma is similar to that in the thymic development process, and what role this relationship plays in the pathogenesis of thymoma.
In this study, we initially examined Wnt4 and FoxN1 mRNA and protein expression in thymoma, and then analyzed the correlation between them. Second, we investigated the regulatory relationship between Wnt4 and FoxN1 and the effect on thymoma cell apoptosis of downregulating their mRNA expression in thymoma cells by siRNA. Finally, we studied the inhibition of thymoma growth upon downregulating Wnt4 and FoxN1 in an animal experiment. We hope that our research will lead to new therapeutic targets of thymoma.
Methods
Sample source and cell culture
Fifty-six thymoma samples were collected from patients who had undergone surgery but had not received chemotherapy or radiotherapy at Tianjin Medical University General Hospital (Tianjin, China) in 2012 to 2015. These included 6 type A thymomas, 8 type AB thymomas, 10 type B1 thymomas, 11 type B2 thymomas, 12 type B3 thymomas, and 9 type C thymomas, according to the WHO histological classification, and 18 stage I, 22 stage II, 12 stage III, and 14 stage IV thymomas, according to Masaoka staging. The ethics committee of Tianjin Medical University General Hospital approved this study.
A human thymoma cell line named Thy0517 was derived in vitro from a 50-year-old Chinese man who had an AB type thymoma and myasthenia gravis (Patent Number: ZL 2014 1 0312866.6, SIPO of the P.R.C). This cell line was maintained and cultured at Tianjin Medical University General Hospital in DMEM supplemented with 10% fetal bovine serum, 100 IU/mL penicillin, and 100 µg/mL streptomycin, in a 37 °C, 5% CO2 humidified atmosphere (9).
Immunohistochemical analysis of Wnt4 and FoxN1 proteins in thymoma
Paraffin-embedded thymoma tissue sections (4 µm) were deparaffinized, dehydrated, and subjected to antigen retrieval with Tris-EDTA under high temperature and high pressure for 3 min. Three sections from the same sample were incubated with rabbit anti-human Wnt4 polyclonal antibody (1:50 dilution; Abcam, Cambridge, UK) and goat anti-human FoxN1 polyclonal antibody (1:50 dilution; Abcam) and a PBS blank overnight at 4 °C. The sections were incubated with goat anti-rabbit secondary antibody, and then were stained with DAB and hematoxylin. Brown granules were considered to represent a positive signal. We scored the intensity of staining as follows: 0, no reaction found; 1, weak staining; 2, moderate staining; 3, strong staining, and the extent of staining as follows: 1, <25%; 2, 25% to <50%; 3, 50% to 75%; 4, >75%. The product of two results was greater than 3 for positive cases. The average frequency of positive cells in five high-power fields (×400) of each section was determined.
Real-time quantitative PCR (RT-qPCR) analysis of thymoma Wnt4 and FoxN1 mRNA
RNA from thymoma tissue was extracted by homogenization of tissue in TRIzol (Invitrogen-Life Technologies, Carlsbad, CA, USA) solution, in accordance with the manufacturer’s instructions. Total RNA (2 µg) was reverse-transcribed into cDNA. cDNA was amplified with SYBR Premix Ex Taq™ (Takara Biotechnology, Dalian, China). The sequences of the PCR primer pairs for Wnt4, JNK, FoxN1, and GAPDH were designed by GeneRunner, as shown in Table 1 (Aoke Biological Technology LLC, Beijing, China). The amplification was performed at 50 °C for 2 min and 95 °C for 1 s for predenaturation; 40 cycles of 94 °C for 30 s for denaturation, 56 °C for 15 s for annealing, and 72 °C for 10 s for extension; followed by a final extension at 72 °C for 5 min. The relative expression levels of target genes were determined using the 2−ΔΔCq method.
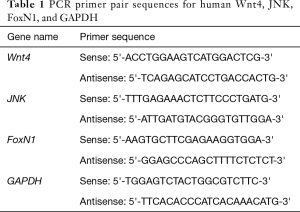
Full table
Construction and selection of Wnt4 siRNA, JNK siRNA, and FoxN1 siRNA
For each of Wnt4, JNK, and FoxN1, three alternative types of siRNA were designed by Shanghai Gene Pharma Co., Ltd. (Shanghai, China). Cells were divided into five groups for each of Wnt4, JNK, and FoxN1 as follows (n=6): (I) control, no interference; (II) Lipo200, no siRNA; (III) siRNA-1; (IV) siRNA-2; and (V) siRNA-3. Thymoma cells were inoculated in six-well culture plates and grown to 70% confluence before transfection. Five microliters of siRNA was transfected for each siRNA group using Lipofectamine™ 2000 (Invitrogen) for 6 h. Cells were then cultured in DMEM with 10% FBS for 48 h and total RNA was extracted for PCR. The relative expression levels of Wnt4, JNK, and FoxN1 mRNA were calculated and compared with those before transfection in the same cell line. The siRNA associated with the best inhibition was selected for subsequent experiments.
RT-qPCR and western blot assay for FoxN1 mRNA and protein expression after downregulating Wnt4 and JNK
Thymoma cells were divided into three groups for determining FoxN1 mRNA and protein expression after downregulating Wnt4 and JNK as follows: (I) control, no interference; (II) Lipo200, no siRNA; and (III) siRNA, siRNA transfection. Reactions were performed in triplicate. Total RNA extraction and RT-qPCR analysis for FoxN1 mRNA were performed 48 h after transfection. FoxN1 protein was analyzed by western blotting using goat anti-human FoxN1 polyclonal antibody (1:50 dilution, Abcam).
Annexin V-FITC/PI double staining and flow cytometry
Thymoma cells were divided into four groups (n=6) as follows: control group, Lipo2000 group, Wnt4 downregulation group, and FoxN1 downregulation group. Apoptosis was detected by flow cytometry with Annexin V-FITC/PI (Chongqing Sanjian Biological Technology Co., Ltd., Chongqing, China) double staining, which distinguishes between apoptotic and dead cells based on differential membrane staining. Different cells can be distinguished from a four-quadrant diagram as follows: normal live cells, Annexin V−/PI− (Q3); viable apoptotic cells, Annexin V+/PI− (Q4); advanced apoptotic cells, Annexin V+/PI+ cells (Q2); and dead cells, Annexin V-/PI+ cells (Q1). We determined the percentage apoptosis by statistical analysis of the Q4 data.
Transplantation and tumor growth in nude mice
Twenty-four BALB/c nude mice were divided into four groups as follows: control group, Lipo2000 group, Wnt4 downregulation group, and FoxN1 downregulation group. The general condition of the nude mice was good after breeding in a specific pathogen-free animal room for three days. Thymoma cells were collected after transfection and inoculated into the right armpit of nude mice on the fourth day. The general condition and tumor growth of the nude mice were observed after inoculation. Tumor volume [V (mm3) = 0.5 × short diameter × long diameter2] was measured and recorded every three days. After 4 weeks, all nude mice were sacrificed and the tumor tissues were weighed and sectioned for histological analysis.
Statistical analysis
The data are presented as mean ± standard deviation (SD). Statistical analysis was conducted using the t-test for paired samples, single-factor analysis of variance (one-way ANOVA) for multi-group data, and Chi-square test for categorical data. Spearman’s rank correlation was used to analyze the relationship of Wnt4 and FoxN1. P values <0.05 were considered statistically significant. Data were processed using SPSS 19.0 (IBM, Chicago, IL, USA).
Results
Wnt4 and FoxN1 protein expression in thymoma
The rate of positive staining of Wnt4 protein in thymoma was 64.3% (36/56), when brown granules in the membrane were considered to represent a positive signal. The positive staining rate of FoxN1 protein in thymoma was 58.9% (33/56), when brown granules in the nucleus were considered to represent a positive signal (Figure 1). A correlation between Wnt4 and FoxN1 protein expression (r=0.438, P=0.01, Table 2) was also identified.
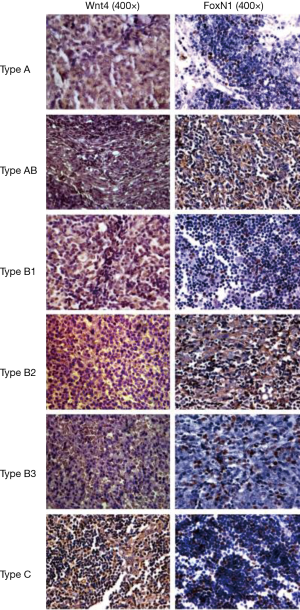
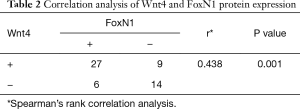
Full table
There were also correlations between Wnt4 and FoxN1 protein expression and the WHO histological classification of thymoma. With increased malignancy, the positive expression rate for both proteins was higher (P<0.05, Table 3). The same results were also obtained when using Masaoka stages (P<0.05, Table 4).

Full table
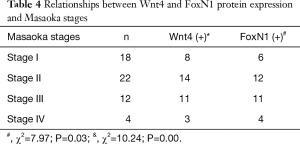
Full table
Wnt4 and FoxN1 mRNA expression in thymoma
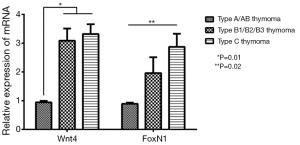
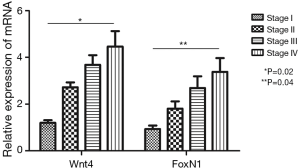
When six cases of normal thymus tissue were used as controls, the relative expression levels of Wnt4 and FoxN1 mRNA in thymoma were 2.56±0.04 and 1.83±0.11, compared with those in normal thymus (P<0.01). There were also correlations between Wnt4 and FoxN1 mRNA expression and the WHO histological classification of thymoma. With increased malignancy, the relative expression was higher (P<0.05, Figure 2). The same results were also obtained when using the Masaoka stages (P<0.05, Figure 3).
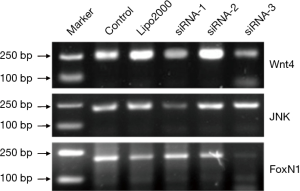
Screening of Wnt4 siRNA, JNK siRNA, and Foxn1 siRNA
The mRNA expression was determined by semiquantitative RT-PCR. Among the three alternative siRNAs for each group, the one with the best interfering effect in the Wnt4 group was Wnt4-siRNA-3, for which the average inhibition rate was 56.7%. The siRNA with the best interfering effect in the JNK group was JNK-siRNA-1, for which the average inhibition rate was 72.6%. The siRNA with the best interfering effect in the FoxN1 group was FoxN1-siRNA-3, for which the average inhibition rate was 63.2% (Figure 4). Therefore, Wnt4-siRNA-3, JNK-siRNA-1, and FoxN1-siRNA-3 were selected as the siRNAs with the best interference effects and used in subsequent experiments.
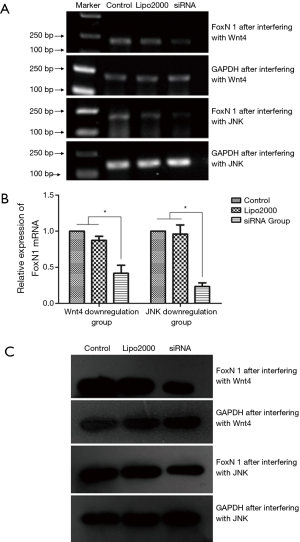
The inhibitory effect on FoxN1 upon interfering with Wnt4 and JNK expression in thymoma cells
After Wnt4-siRNA-3 had been transfected into the thymoma cell line (Thy0517), the relative expression of Foxn1 mRNA decreased (0.42±0.19) compared with that in the Lipo2000 group (0.87±0.10) and the control group (P<0.05, Figure 5A,B). In addition, after JNK-siRNA-1 transfection, the relative expression of Foxn1 mRNA decreased (0.23±0.09), compared with that in the Lipo2000 group (0.96±0.22) and the control group (P<0.05, Figure 5A,B). Western blot results showed that the expression of FoxN1 protein in the siRNA group was significantly lower than that in the control and Lipo2000 groups after Wnt4-siRNA-3 or JNK-siRNA-1 transfection (Figure 5C). These results indicate that the transfection of Wnt4-siRNA-3 or JNK-siRNA-1 could inhibit FoxN1 mRNA and protein expression.
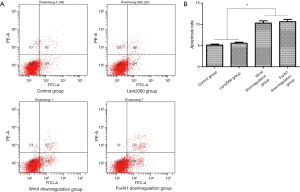
Detection of apoptosis after Wnt4 and FoxN1 downregulation by flow cytometry
The apoptosis rates in the Wnt4 downregulation group and the FoxN1 downregulation group were 10.33%±0.51% and 10.65%±0.55%, respectively, which were lower than those in the control group (5.12%±0.23%) (P<0.01) and the Lipo2000 group (5.58%±0.23%) (P<0.01). The Lipo2000 group did not differ compared with the controls (P>0.05) (Figure 6).
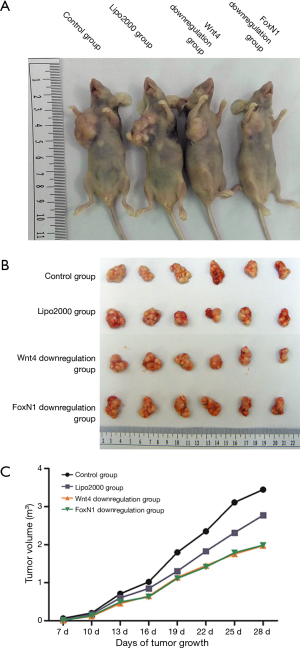
Tumor growth in nude mice
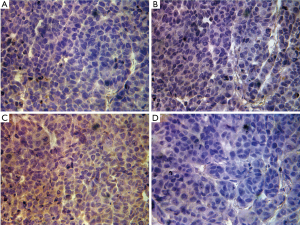
All nude mice were examined for tumor formation and the speed of tumor growth on the third day after inoculation. Nude mice were sacrificed at the end of 4 weeks after tumor formation. The tumor volumes of the Wnt4 downregulation group and the FoxN1 downregulation group were 1.97±0.57 and 1.99±0.51 cm3, respectively, compared with 3.44±1.21 cm3 in the control group (P=0.007), while the level was 2.77±0.57 cm3 in the Lipo2000 group (P=0.117 and 0.122). There was also no significant difference in volume between the control group and the Lipo2000 group (P=0.18). The weights of tumor in the control group, Lipo200 group, Wnt4 downregulation group, and FoxN1 downregulation group were 2.1±0.67, 1.75±0.39, 1.48±0.48, and 1.46±0.35 g, respectively, which did not differ significantly (P>0.05) (Figure 7). Immunohistochemical analysis of Wnt4 and FoxN1 protein expression in tumor showed Wnt4 protein diffusely expressed in FoxN1 downregulation group, but not in Wnt4 downregulation group. Whereas FoxN1 protein was scarcely expressed in these two groups (Figure 8). This indicated that siRNA interfered Wnt4 and FoxN1 successfully.
Discussion
Wnt4 and FoxN1 are expressed in thymic epithelial cells. They play a key role in the development of the thymus, and their expression decreases during or after thymic atrophy (10,11). The overexpression of Wnt4 and FoxN1 may affect thymic atrophy or promote the proliferation of thymic epithelial cells, leading to thymoma development. Nonaka and colleagues characterized FoxN1 protein expression by an immunohistochemical assay in 58 cases of thymoma and 17 cases of thymic carcinoma. FoxN1 was diffusely expressed in the nucleus in all cases of type B and AB thymoma and in all but one case of type A thymoma, whereas the expression was generally focal in thymic carcinoma (76%) (12). Weissferdt characterized FoxN1 protein expression in 65 cases of thymic carcinoma and obtained similar results, with the rate of FoxN1 positivity being 68% (13). Wnt4 has also been reported to play a role in the pathogenesis of breast cancer and hepatocellular carcinoma (14,15), but its expression in thymoma has seldom been reported.
Our results showed that thymoma not only exhibited abnormal expression of Wnt4 and FoxN1 mRNA and protein, but also that their expression levels increased with increasing WHO histological thymoma classification and Masaoka stage. The WHO classification and Masaoka stage reflect the degree of malignancy and the prognosis of thymoma to a certain extent, so our findings suggest that the expression levels of Wnt4 and FoxN1 are related to the malignancy and prognosis of thymoma. In addition, Wnt4 and FoxN1 protein expression levels were positively correlated, indicating that they may present some relationship in thymoma.
Both Wnt4 and FoxN1 are required for thymic epithelial patterning, differentiation, and proliferation; specifically, FoxN1 induces these processes under the control of Wnt4 (16). Thymic epithelial Wnt4 secretion and FoxN1 expression were also reported to decrease with thymic senescence (17). We used the thymoma cell line Thy0517 to study the relationship between Wnt4 and FoxN1 in thymoma in vitro. Our previous study suggested that Wnt4 plays a role in thymoma through a non-classical pathway mediated by JNK, rather than the classical pathway mediated by β-catenin. Therefore, in this study, we inhibited Wnt4 and JNK gene expression by siRNA interference. With the decrease of Wnt4 and JNK expression, the expression of FoxN1 mRNA and protein decreased. This showed that Wnt4 plays a role in regulating FoxN1, and that the Wnt4 gene is upstream of FoxN1 in thymoma, exerting its effect by regulating the expression of FoxN1 downstream of it.
During the development of the thymus, normal expression levels of Wnt4 and FoxN1 are critical for maintaining the balance between TEC proliferation and apoptosis; activation of the Wnt4 signaling pathway has also been shown to promote TEC proliferation (18,19). Therefore, downregulation of the high expression of Wnt4 and FoxN1 in thymoma cells could inhibit tumor growth and promote cell apoptosis. We confirmed this using an apoptosis assay by applying flow cytometry and tumor inoculation in nude mice. The experimental results showed that the downregulation of Wnt4 and FoxN1 promoted the apoptosis of thymoma cells and reduced the tumor volume. However, the apoptosis rate was still too low to be considered to have a substantial therapeutic effect, at only about 15%, and the weight of the tumor was not impacted too markedly. Therefore, we assumed that there are other signaling pathways that affect the proliferation and apoptosis of thymoma, and that the inhibition of Wnt4-FoxN1 alone can only play a small role in restraining thymoma growth.
In conclusion, the overexpression of Wnt4 and FoxN1 was found in each type of thymoma, and their expression levels were found to correlate with the malignancy of thymoma. Wnt4 was also correlated with FoxN1 expression. As an upstream factor, the Wnt4 signaling pathway can regulate the expression of FoxN1 at the gene and protein levels. In addition, the apoptosis of thymoma cells increased and tumor growth was inhibited after the downregulation of Wnt4 and FoxN1. This study sheds some light on the pathogenesis of thymoma development and provides a theoretical basis for treating thymoma using Wnt4 and FoxN1 as targets.
Acknowledgements
Funding: This study was partly supported by a grant from the Key Project from National Natural Science Foundation of Tianjin (No. 14JCZDJC57100), the Major Project of Science and Technology of Tianjin (No. 16ZXMJSY00030).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The ethics committee of Tianjin Medical University General Hospital approved this study.
References
- Rode I, Martins VC, Kublbeck G, et al. Foxn1 protein expression in the developing, aging, and regenerating thymus. J Immunol 2015;195:5678-87. [Crossref] [PubMed]
- Palamaro L, Romano R, Fusco A, et al. FOXN1 in organ development and human diseases. Int Rev Immunol 2014;33:83-93. [Crossref] [PubMed]
- Shin M, Nagai H, Sheng G. Notch mediates Wnt and BMP signals in the early separation of smooth muscle progenitors and blood/endothelial common progenitors. Development 2009;136:595-603. [Crossref] [PubMed]
- Osada M, Jardine L, Misir R, et al. DKK1 mediated inhibition of Wnt signaling in postnatal mice leads to loss of TEC progenitors and thymic degeneration. PLoS One 2010;5:e9062. [Crossref] [PubMed]
- Balciunaite G, Keller MP, Balciunaite E, et al. Wnt glycoproteins regulate the expression of FoxN1, the gene defective in nude mice. Nat Immunol 2002;3:1102-8. [Crossref] [PubMed]
- Kvell K, Fejes AV, Parnell SM, et al. Active Wnt/beta-catenin signaling is required for embryonic thymic epithelial development and functionality ex vivo. Immunobiology 2014;219:644-52. [Crossref] [PubMed]
- den Bakker MA, Roden AC, Marx A, et al. Histologic classification of thymoma: a practical guide for routine cases. J Thorac Oncol 2014;9:S125-30. [Crossref] [PubMed]
- Zhang H, Zhang P, Liu Y, et al. In vitro study of the effect of small interfering ribonucleic acid on the expression of FOXN1 and B cell-attracting chemokine 1 in thymoma cell lines. Thorac Cancer 2015;6:172-9. [Crossref] [PubMed]
- Wang G, Wang Y, Zhang P, et al. Establishment and characterization of a novel cell line derived from thymoma with myasthenia gravis patients. Thorac Cancer 2015;6:194-201. [Crossref] [PubMed]
- Talaber G, Kvell K, Varecza Z, et al. Wnt-4 protects thymic epithelial cells against dexamethasone-induced senescence. Rejuvenation Res 2011;14:241-8. [Crossref] [PubMed]
- Pan B, Liu J, Zhang Y, et al. Acute ablation of DP thymocytes induces up-regulation of IL-22 and Foxn1 in TECs. Clin Immunol 2014;150:101-8. [Crossref] [PubMed]
- Nonaka D, Henley J D, Chiriboga L, et al. Diagnostic utility of thymic epithelial markers CD205 (DEC205) and Foxn1 in thymic epithelial neoplasms. Am J Surg Pathol 2007;31:1038-44. [Crossref] [PubMed]
- Weissferdt A, Moran CA. Thymic carcinoma, part 1: a clinicopathologic and immunohistochemical study of 65 cases. Am J Clin Pathol 2012;138:103-14. [Crossref] [PubMed]
- Sikora MJ, Jacobsen BM, Levine K, et al. WNT4 mediates estrogen receptor signaling and endocrine resistance in invasive lobular carcinoma cell lines. Breast Cancer Res 2016;18:92. [Crossref] [PubMed]
- Wolfe A, Thomas A, Edwards G, et al. Increased activation of the Wnt/β-catenin pathway in spontaneous hepatocellular carcinoma observed in farnesoid X receptor knockout mice. J Pharmacol Exp Ther 2011;338:12-21. [Crossref] [PubMed]
- Reis MD, Csomos K, Dias LP, et al. Decline of FOXN1 gene expression in human thymus correlates with age: possible epigenetic regulation. Immun Ageing 2015;12:18. [Crossref] [PubMed]
- Kvell K, Varecza Z, Bartis D, et al. Wnt4 and LAP2alpha as pacemakers of thymic epithelial senescence. PLoS One 2010;5:e10701. [Crossref] [PubMed]
- Wei T, Zhang N, Guo Z, et al. Wnt4 signaling is associated with the decrease of proliferation and increase of apoptosis during age-related thymic involution. Mol Med Rep 2015;12:7568-76. [PubMed]
- Vaidya HJ, Briones Leon A, Blackburn CC. FOXN1 in thymus organogenesis and development. Eur J Immunol 2016;46:1826-37. [Crossref] [PubMed]


