Malignant pleural mesothelioma: current and future perspectives
Introduction
Malignant pleural mesothelioma (MPM) represents a common malignant disease. It is an aggressive tumor arising from the mesothelial cells lining the pleura (1). There is an extremely poor prognosis and a vast majority of MPM patients are diagnosed in an advanced stage. Rapid progression of the disease, no effective therapeutic approach and resistance to chemotherapy and radiotherapy resulted in a median survival time of less than 12 months (2).
Exposure to airborne asbestos fibers is mainly associated with the development of MPM (3). Incidences of MPM reach 100 cases/million/year in occupationally exposed populations opposed to 1 case/million/year in the general population (4). Wagner and his colleagues were the first to describe the relationship between asbestos and MPM in 1960 when he published a series of MPM cases in asbestos mine workers from South Africa (5). Western Europe (6,7), United States (8), Japan (9), Australia (10), India (11), China, Indonesia and Vietnam (12) include countries where the incidents of MPM are expected to increase. This prediction is supported by the extensive use of asbestos in developed countries since 1950s and the continuing use in developing countries considering that the incubation period between initial exposure to asbestos and MPM diagnosis is 20 to 50 years (13). However, radiation, exposure to other mineral fibers such as erionite, simian virus 40 and genetic predisposition (14-16) include also causative agents for the development of MPM.
Although 50 years have passed since the discovery of the first incidence of MPM, an optimal strategy has not been yet established, as the diagnosis, staging and treatment of the disease remains difficult and complex. Recent advances in the field of genetic and molecular biology of cancer as well as in immunohistochemistry techniques have led to an improved identification and understanding of the tumor phenotypes. This individual approach is generally termed as ‘personalized medicine’ (17). However, to date there are no established indicators of clinical significance in MPM. In the present review, we will summarize the existing knowledge of the MPM management reporting the most clinically useful and promising prognostic factors of MPM.
Pathogenesis and diagnosis
Due to occupational exposure, MPM is more common in men than in women (5:1 ratio) (18) and more frequent in advanced ages as a result of the long latency period. The most common symptoms are shortness of breath and pain (90%) while others include tiredness (36%), worry (29%), cough (22%), sweating (22%) and constipation (22%) (19).
MPM is divided into three major histological sub-types: sarcomatoid biphasic and epithelioid. Epithelioid is the most common sub-type among patients with MPM (<50%), associated also with the best prognosis (20). Diagnostic procedures can be either non-invasive such as Chest X-ray, CT, FDG-PET or invasive such as image-guided (CT or US) pleural biopsy, extrapleural pneumonectomy (EPP) or laparoscopy (20). Video-assisted thoracoscopy is the best biopsy technique (accuracy of 98%) and cytology, a reliable diagnostic tool for experienced cytopathologists, can offer additional tissue confirmation. Thus, several immunohistochemical panels are proposed to distinguish between sub-types of mesothelioma, secondary carcinoma and other malignant tumors metastatic to serosal membranes (21). Calretinin is the most commonly used antibody, positive for mesothelioma with a reported sensitivity of 95% and specificity of 87% (22). Other useful antibodies include thrombomodulin, mesothelin and cytokeratin 5 (22). It is recommended by the International Mesothelioma Interest Group that the immunohistochemical markers have either sensitivity or specificity greater than 80% (23). However, as no mesothelial marker has 100% sensitivity and specificity for mesothelioma diagnosis, the need to identify new panels is crucial. To date, no tissue or serum marker has been shown to have sufficient specificity, consistency and reproducibility (21). Also, given that the disease is infrequent and only a few pathologists have extensive experience with mesothelioma, make the diagnosis more difficult.
Molecular genetic analysis has revealed three key genetic alterations that can lead to the development of new diagnostic tools and new target therapies. Cyclin-dependent kinase inhibitor 2A/alternative reading frame (CDKN2A/ARF), neurofibromatosis type 2 (NF2) and BRCA1-associated protein-1 (BAP1) genes are the most frequently mutated tumor suppressor genes that can be detected in malignant mesothelioma cells (24).
Up to date, several staging systems for mesothelioma have been used but were proven inadequate to improve therapeutic outcomes. The most practical and most commonly used system is the tumor-node-metastasis system developed by the International Mesothelioma Interest Group (23). Other staging systems include the Butchart system which is the oldest one and the Brigham system which is currently not used (25).
Treatment
Treatment of MPM can be classified into radical procedures such as surgery and into palliative measures which concern the removal of pleural effusions and the preventing of their recurrence in order to relieve the symptoms such as dyspnea and chest pain. Some researchers suggested that the radical procedures had a better prognosis (26), however, later studies could not confirm this suggestion (27,28). Today, once the diagnosis is made there are no accepted or published guidelines to establish a standard surgical approach. It is a fact that surgery is not an option for the majority of the patients due to the diffuse spreading growth of this neoplasm (29).
Apart from the controversy on whether surgery increases survival, another issue is the lack of evidence in comparing the commonly used techniques such as EPP and pleurectomy/decortication (P/D) in multi-institutional, randomized-controlled trials (30). Nevertheless, according to Mesothelioma and Radical Surgery (MARS), a multicentre randomised controlled trial (28), MPM patients treated with P/D had an equal to better outcome than those treated with EPP which raised a question whether performing a P/D with perioperative chemotherapy would have better outcome with a lower operative mortality than EPP and perioperative chemotherapy (31,32). However, these techniques are not suitable for the majority of the patients due to locally advanced or unresectable disease (33). Several factors should be taken into account concerning the choice of surgery treatment such as disease stage, the patient’s cardiopulmonary reserve, surgeon’s experience and the extent of planned adjuvant therapy (30). Another surgical approach includes lung-sparing cytoreductive surgery which is usually combined with chemotherapy and radiation (trimodality treatment) (34). In a systematic review conducted by The et al., results of 1,270 patients from 26 studies were analyzed (34). The authors suggested that more controlled trials would lead to further consideration of lung-sparing cytoreductive surgery.
However, since the role of surgery as single-modality therapy in MPM remains controversial, the management of MPM consists of combinations between platinum-based chemotherapy, surgery and radiation. Similarly to surgical treatment, there is also no evidence of survival benefit concerning radical radiotherapy of the hemithorax when compared to best supportive care (35). Radiotherapy is mainly applied as adjuvant treatment or for symptom relief (36). Hence, radiotherapy has proven to be a disappointment in the management of MPM.
Currently, multimodality strategies include EPP or pleurectomy combined with adjunctive therapies such as immunotherapy, chemotherapy and radiotherapy which are used on a case by case basis, however, frequently the only choice available is palliative treatment (37). As far as immunotherapy is concerned, in spite of favorable results in vitro and in vivo in animal models (38-40), in clinical trials limited success was achieved (41-43).
Chemotherapy in MPM patients includes an option for resectable and unresectable tumors. However, MPM patients appear to be resistant to chemotherapy due to epigenetic errors leading to inadequate gene expression in tumor cells, consequently novel strategies are expected to arise concerning epigenetic therapies (44).
Chemotherapy in MPM patients is given either as single agent treatment or in most cases combination of drugs which has shown improved response rates and survival. Vogelzang et al. conducted a phase III clinical trial of 456 MPM patients comparing cisplatin plus pemetrexed to cisplatin alone reporting superior survival time of 2.8 months, time to progression and response rates for the combination (45) (Table 1). After the results of this study, cisplatin in combination with pemetrexed has been established as standard first-line treatment for MPM patients in advanced stage disease (50). However, this combination confers a median progression-free survival (PFS) of 5.7 months and there is no alternative when MPM patients fail this treatment option (51).
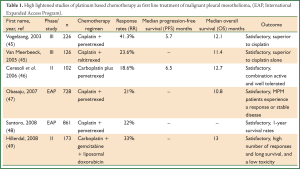
Full Table
Other efforts which reported promising results include studies which combined carboplatin with pemetrexed in MPM (46-48,52). The use of gemcitabine, another antitumor agent, has been limited in rather small size clinical trials and its efficacy in cancer as a single-agent has shown to be either unsatisfactory (53,54) or sufficient in combination with cisplatin (55-57).
Furthermore, a phase II clinical trial concluded that raltitrexed, a thymidine synthase inhibitor as a single agent had activity in MPM patients (58). Similarly, in a more recent phase III trial, it was demonstrated a 2.6-month improvement in OS, 11.4 months for the combination of raltitrexed and cisplatin, compared to 8.8 months for cisplatin alone (50).
To date, there is no standard second-line treatment for MPM. Phase III clinical trials reported feasible results, when pemetrexed alone or combined with cisplatin was compared in patients who had previously received systemic chemotherapy (59,60). More recently, Bearz et al. concluded that if a patient had a long-lasting benefit from previous treatment with pemetrexed combined with a cisplatin compound, the same treatment should be offered at progression (61).
Another drug that has been investigated in clinical trials of MPM is vinorelbine which has already shown satisfactory results in breast cancer (62) and non-small cell lung carcinoma (NSCLC) (63). In a phase II clinical trial it was suggested that due to the relatively low toxicity of vinorelbine, the combination of this drug with other agents should be feasible (64). Moreover, Muers et al. conducted a multicenter randomized trial (MS01) in which active symptom control (ASC) with or without chemotherapy in the treatment of patients with MPM was analyzed (19). The researchers concluded that the addition of chemotherapy to ASC offered no significant benefits in terms of OS or quality of life, but exploratory analyses suggested that vinorelbine merited further investigation.
More recently, Sorensen et al. reported that cisplatin and intravenous vinorelbine was a highly active regimen in MPM with a response rate and survival comparable to the most active regimens so far reported (65) while Stebbing et al. evaluated the efficacy and safety of weekly vinorelbine in relapsed MPM patients reporting a reasonable response rate with an acceptable toxicity profile in the second-line treatment of MPM (66).
Despite the positive results regarding the combination of doxorubicin, an active drug for MPM patients, with cisplatin during phase II studies, long-term use is not an option due to its toxicity profile (67-69). In contrast, liposomal doxorubicin (LD), an agent with different toxicity profile was evaluated in phase II trials in combination with cisplatin (70) or with carboplatin and gemcitabine (49). The authors identified them as active combinations for MPM treatment with acceptable toxicity profile. However, phase III trials should be conducted to compare LD plus cisplatin to cisplatin/pemetrexed or cisplatin/raltitrexed for the determination of standard first line treatment.
Currently, the most aggressive multimodality treatment includes chemotherapy, post-operative radiotherapy and surgery. Recent studies demonstrated that patients completing trimodality treatment had a median survival of 29 months (71,72). However, the European Organisation for Research and Treatment of Cancer (EORTC; protocol 08031) phase II trial investigated the feasibility of trimodality therapy consisting of induction chemotherapy (cisplatin + pemetrexed) followed by EPP and post-operative radiotherapy in MPM patients (27). Although the results were positive, trimodality therapy was not completed within the strictly defined timelines of this protocol and adjustments were necessary. A similar approach which was conducted in a small study of 36 patients (73) failed to show any survival benefit. Thus, there are limited results regarding the trimodality treatment, applicable only at a very early stage of MPM patients with a good performance status (71). Therefore, more data from multicenter randomized clinical trials are needed.
Targeted treatments and biomarkers
MPM treatment is guided mainly by clinical stage and patient characteristics and not by histological or molecular features of the tumor. Moreover, platinum based chemotherapies and available treatments have failed to show improvements in survival benefits and there are no other approved regimens for relapsed or refractory MPM. However, the expanding knowledge on molecular mechanisms has led to the identification of several novel targets and biomarkers. Molecular pathways that have been identified in MPM include cell cycle regulation, apoptosis, growth factor pathways and angiogenesis (74).
More specifically, up-regulation of epidermal growth factor receptor (EGFR) is an important part of MPM development, thus, EGFR-tyrosine kinase inhibitors (TKIs) such as ZD1839 (gefitinib) and OSI-774 (erlotinib) might represent novel therapeutic options. In vitro studies have shown that gefitinib inhibited MPM cell growth and survival preventing EGF-dependent activation of ERK1/2 pathway by blocking EGFR-TK phosphorylation and stabilizing inactive EGFR dimers (75,76).
Furthermore, recent studies have identified that the presence of specific EGFR mutations was predictive of response to therapy and cancer outcome in NSCLC (77). Similarly, EGFR activating mutations in mesothelioma were recently identified for the first time appearing to share same ‘relative’ improved clinical outcome like mutant EGFR-NSCLC (78).
Vascular endothelial growth factor (VEGF) signaling also plays a very important role in MPM. Several angiogenesis inhibitors have been used in clinical trials such as bevacizumab (Avastin; Genentech, South San Francisco, CA), a recombinant humanized monoclonal antibody, or other antiangiogenic agents SU5416, vatalanib, thalidomide and sorafenib which have shown modest activity as single-agent treatments; Thus, further research is needed to conduct comparisons with other agents (79).
In a recent multicenter randomized phase II trial, the addition of bevacizumab to gemcitabine/cisplatin was evaluated but it did not manage to improve significantly PFS or OS in malignant mesothelioma patients (80) (Table 2). Currently, several studies of bevacizumab in combination with pemetrexed and cisplatin are ongoing (http://www.clinicaltrials.gov) and it is expected that antiangiogenic therapy could benefit subgroups of MPM patients (84).
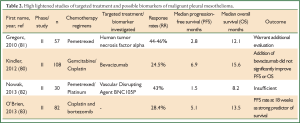
Full Table
Recently, Nascreen et al. in their review included receptor EphA2 as a novel potential molecular target in MPM (18). Other inhibitors include histone deacetylase (HDAC) inhibitor which plays a role in cellular differentiation and malignant transformation of MPM. HDAC has shown a partial response in a phase I trial (85). Met signaling pathway is also a very promising target of MPM for patients expressing both Met and HGF, as selective small molecular inhibitors of c-Met kinase were shown to be effective in vitro and in vivo experiments (86). Another promising drug is ranpirnase, a ribonuclease (RNase) isolated from early embryos of the Northern Leopard Frog (87), which proved to have disease-modifying activity against malignant mesothelioma (88). A potent antitumor agent is vandetanib which markedly enhanced pemetrexed and carboplatin activity against established MPM cell lines (89).
In addition, a phase II study of asparagine-glycine-arginine-human tumor necrosis factor alpha (NGR-hTNF), a selective vascular targeting agent, in previously treated patients with MPM found modest results warranting additional evaluation (81). Modest results were shown in a phase II trial (82) for BNC105P, an inhibitor of tubulin polymerization that has vascular disrupting and antiproliferative effects (90), as second line therapy in MPM after first line pemetrexed/platinum chemotherapy.
According to reports extracellular signal-regulated kinase 5 ERK5 inhibition in combination with chemotherapeutic drugs is a beneficial strategy for combination therapy in patients with malignant mesothelioma (91). Another study combined negative ERCC1 and class III β-tubulin immunostaining to be associated with significantly prolonged PFS and OS in MPM patients receiving cisplatin-vinorelbine therapy (92).
In a prospective phase II study of cisplatin and bortezomib (CB) a protease inhibitor, as first line treatment of MPM was investigated. The researchers reported validation of progression free survival rate at 18 weeks (PFSR-18) as primary end-point which was confirmed as a strong predictor of survival (83).
The lack of established biomarkers in MPM makes difficult to achieve positive outcomes of targeted agents in clinical trials, however, several efforts have been reported (Table 3). The first study to suggest serum mesothelin (a cell surface glycoprotein on normal mesothelial cells) as a biomarker of mesothelioma was reported in 2003 by Robinson et al. (93) using an enzyme-linked immunosorbent assay (ELISA) which was later commercialized as Mesomark (Fujirebio Diagnostics, Malvern, PA) and was approved in 2007 by the US Food and Drug Administration (94). However, the main limitation of mesothelin is its poor sensitivity, which makes difficult to achieve early diagnosis (95). Furthermore, N-ERC/mesothelin (N-ERC) index is considered to be a useful biomarker for predicting not only the chemotherapeutic response but also the prognosis in patients with advanced MPM (108). Another glycoprotein is osteopontin whose baseline levels were proven to be an independent negative predictor of MPM survival, however, they were less associated to the disease than mesothelin serum levels (96). A similar study also demonstrated that osteopontin had a lower diagnostic accuracy than mesothelin in patients suspected of MPM (97).
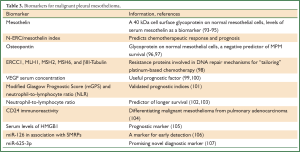
Full Table
Resistance proteins involved in DNA repair mechanisms such as ERCC1, MLH1, MSH2, MSH6, and βIII-Tubulin are found to be associated with response and outcome to platinum-based chemotherapy in MPM patients (98). Thus, these enzymes could be used as biomarkers for “tailoring” platinum-based chemotherapy for MPM patients who may expect the largest clinical benefit.
VEGF serum concentration could also be a useful prognostic factor, as suggested in recent studies (99,100). More specifically, 51 MPM patients were found with significantly higher serum levels of VEGF when compared to 42 individuals with benign asbestos-related diseases (asbestosis or pleural plaques) or who were healthy despite asbestos exposure (100). Similarly, in another study it was demonstrated that patients with MPM had significantly higher pleural effusion VEGF levels than a population with non-malignant pleuritis or lung cancer involving malignant pleural effusion (99).
It is known that chronic inflammation plays a key role in the pathogenesis of MPM and recently the inflammation-based prognostic scores such as modified Glasgow Prognostic Score (mGPS) and neutrophil-to-lymphocyte ratio (NLR) were found to be externally validated prognostic indices in 171 MPM patients (101). NLR was also suggested as an independent predictor of longer survival for patients with MM undergoing systemic therapy (102) or as a poor prognostic factor in patients undergoing EPP (104).
Recently, CD24 immunoreactivity was identified as potential new marker in differentiating malignant mesothelioma from pulmonary adenocarcinoma (104). Other researchers investigated serum levels of HMGB1 in MPM patients comparing them with a population previously exposed to asbestos without developing MPM (105), suggesting it as prognostic marker for MPM. As soluble mesothelin-related peptides (SMRPs) have been suggested as promising biomarkers for MPM (109) and microRNAs (miRNAs) have shown to be involved in cancer (110) and malignant mesothelioma (111), Santarelli et al. proposed miR-126 in association with SMRPs, as a marker for early detection of MPM (106). Moreover, another study suggested miR-625-3p as a promising novel diagnostic marker for MPM (107).
Other researchers evaluated a new combined therapy consisting of ascorbate/epigallocatechin-3-gallate/gemcitabine mixture (called AND, for Active Nutrients/Drug). The authors concluded that this combination was synergistic in vitro on MPM cells, and blocked in vivo tumor progression and metastasization in REN-based xenografts (112). Despite the amount of biomarkers that are being presently investigated, the biological heterogeneity of MPM effects the identification of clinically validated prognostics factors.
Conclusions
In summary, the increasing MPM incidents are a fact, making the need of novel treatments more demanding. Surgery, radiotherapy, and chemotherapy have failed as single modality therapies and first-line standard chemotherapy of MPM, the combination of cisplatin and pemetrexed offers no further improvements in survival. Furthermore, the lack of randomized trials is added to the lack of efficient treatment. Thus, novel therapeutic strategies such as multimodality treatment, targeted agents and improved biomarkers include the on-going research to prolong patient’s survival and quality of life. It is crucial that large clinical trials should be implemented so that efficient and practical serum biomarkers can be identified for the prediction and evolution of the disease.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ismail-Khan R, Robinson LA, Williams CC Jr, et al. Malignant pleural mesothelioma: a comprehensive review. Cancer Control 2006;13:255-63. [PubMed]
- Robinson BW, Musk AW, Lake RA. Malignant mesothelioma. Lancet 2005;366:397-408. [PubMed]
- Stumphius J, Meyer PB. Asbestos bodies and mesothelioma. The Annals of Occupational Hygiene 1968;11:283-93. [PubMed]
- Porret E, Madelaine J, Galateau-Salle F, et al. Epidemiology, molecular biology, diagnostic and therapeutic strategy of malignant pleural mesothelioma in 2007-an update. Rev Mal Respir 2007;24:6S157-64.
- Wagner JC, Sleggs CA, Marchand P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med 1960;17:260-71. [PubMed]
- McElvenny DM, Darnton AJ, Price MJ, et al. Mesothelioma mortality in Great Britain from 1968 to 2001. Occup Med (Lond) 2005;55:79-87. [PubMed]
- Hodgson JT, McElvenny DM, Darnton AJ, et al. The expected burden of mesothelioma mortality in Great Britain from 2002 to 2050. Br J Cancer 2005;92:587-93. [PubMed]
- Larson T, Melnikova N, Davis SI, et al. Incidence and descriptive epidemiology of mesothelioma in the United States, 1999-2002. Int J Occup Environ Health 2007;13:398-403. [PubMed]
- Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med 2005;353:1591-603. [PubMed]
- Leigh J, Davidson P, Hendrie L, et al. Malignant mesothelioma in Australia, 1945-2000. Am J Ind Med 2002;41:188-201. [PubMed]
- Joshi TK, Gupta RK. Asbestos-related morbidity in India. Int J Occup Environ Health 2003;9:249-53. [PubMed]
- Pass HI, Vogelzang N, Hahn S, et al. Malignant pleural mesothelioma. Curr Probl Cancer 2004;28:93-174. [PubMed]
- Marinaccio A, Binazzi A, Cauzillo G, et al. Analysis of latency time and its determinants in asbestos related malignant mesothelioma cases of the Italian register. Eur J Cancer 2007;43:2722-8. [PubMed]
- Testa JR, Carbone M, Hirvonen A, et al. A multi-institutional study confirms the presence and expression of simian virus 40 in human malignant mesotheliomas. Cancer Res 1998;58:4505-9. [PubMed]
- Roushdy-Hammady I, Siegel J, Emri S, et al. Genetic-susceptibility factor and malignant mesothelioma in the Cappadocian region of Turkey. Lancet 2001;357:444-5. [PubMed]
- Carbone M, Albelda SM, Broaddus VC, et al. Eighth international mesothelioma interest group. Oncogene 2007;26:6959-67. [PubMed]
- André F, Ciccolini J, Spano JP, et al. Personalized medicine in oncology: where have we come from and where are we going? Pharmacogenomics 2013;14:931-9. [PubMed]
- Nasreen N, Khodayari N, Mohammed KA. Advances in malignant pleural mesothelioma therapy: targeting EphA2 a novel approach. Am J Cancer Res 2012;2:222-34. [PubMed]
- Muers MF, Stephens RJ, Fisher P, et al. Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): a multicentre randomised trial. Lancet 2008;371:1685-94. [PubMed]
- Pinto C, Novello S, Torri V, et al. Second Italian consensus conference on malignant pleural mesothelioma: state of the art and recommendations. Cancer Treat Rev 2013;39:328-39. [PubMed]
- Henderson DW, Reid G, Kao SC, et al. Challenges and controversies in the diagnosis of mesothelioma: Part 1. Cytology-only diagnosis, biopsies, immunohistochemistry, discrimination between mesothelioma and reactive mesothelial hyperplasia, and biomarkers. J Clin Pathol 2013. [Epub ahead of print]. [PubMed]
- Yaziji H, Battifora H, Barry TS, et al. Evaluation of 12 antibodies for distinguishing epithelioid mesothelioma from adenocarcinoma: identification of a three-antibody immunohistochemical panel with maximal sensitivity and specificity. Mod Pathol 2006;19:514-23. [PubMed]
- Husain AN, Colby T, Ordonez N, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 2013;137:647-67. [PubMed]
- Sekido Y. Molecular pathogenesis of malignant mesothelioma. Carcinogenesis 2013;34:1413-9. [PubMed]
- Richards WG. Recent advances in mesothelioma staging. Semin Thorac Cardiovasc Surg 2009;21:105-10. [PubMed]
- Treasure T, Internullo E, Fiorentino F, et al. A survey of opinions and beliefs concerning surgery for malignant pleural mesothelioma amongst 802 members of the European Association for Cardio-Thoracic Surgery (EACTS), the European Society of Thoracic Surgeons (ESTS) and the Society of Thoracic Surgeons (STS). Interact Cardiovasc Thorac Surg 2011;12:341-6. [PubMed]
- Van Schil PE, Baas P, Gaafar R, et al. Trimodality therapy for malignant pleural mesothelioma: results from an EORTC phase II multicentre trial. Eur Respir J 2010;36:1362-9. [PubMed]
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. [PubMed]
- Treasure T, Sedrakyan A. Pleural mesothelioma: little evidence, still time to do trials. Lancet 2004;364:1183-5. [PubMed]
- Kaufman AJ, Flores RM. Surgical treatment of malignant pleural mesothelioma. Curr Treat Options Oncol 2011;12:201-16. [PubMed]
- Hiddinga BI, van Meerbeeck JP. Surgery in mesothelioma--where do we go after MARS? J Thorac Oncol 2013;8:525-9. [PubMed]
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6, 626.e1-3.
- Sugarbaker DJ, Norberto JJ. Multimodality management of malignant pleural mesothelioma. Chest 1998;113:61S-5S. [PubMed]
- Teh E, Fiorentino F, Tan C, et al. A systematic review of lung-sparing extirpative surgery for pleural mesothelioma. J R Soc Med 2011;104:69-80. [PubMed]
- Alberts AS, Falkson G, Goedhals L, et al. Malignant pleural mesothelioma: a disease unaffected by current therapeutic maneuvers. J Clin Oncol 1988;6:527-35. [PubMed]
- Belli C, Fennell D, Giovannini M, et al. Malignant pleural mesothelioma: current treatments and emerging drugs. Expert Opin Emerg Drugs 2009;14:423-37. [PubMed]
- West SD, Lee YC. Management of malignant pleural mesothelioma. Clin Chest Med 2006;27:335-54. [PubMed]
- Kruklitis RJ, Singhal S, Delong P, et al. Immuno-gene therapy with interferon-beta before surgical debulking delays recurrence and improves survival in a murine model of malignant mesothelioma. J Thorac Cardiovasc Surg 2004;127:123-30. [PubMed]
- Galffy G, Mohammed KA, Nasreen N, et al. Inhibition of interleukin-8 reduces human malignant pleural mesothelioma propagation in nude mouse model. Oncol Res 1999;11:187-94. [PubMed]
- Gattacceca F, Pilatte Y, Billard C, et al. Ad-IFN gamma induces antiproliferative and antitumoral responses in malignant mesothelioma. Clin Cancer Res 2002;8:3298-304. [PubMed]
- Castagneto B, Zai S, Mutti L, et al. Palliative and therapeutic activity of IL-2 immunotherapy in unresectable malignant pleural mesothelioma with pleural effusion: Results of a phase II study on 31 consecutive patients. Lung Cancer 2001;31:303-10. [PubMed]
- Monnet I, Breau JL, Moro D, et al. Intrapleural infusion of activated macrophages and gamma-interferon in malignant pleural mesothelioma: a phase II study. Chest 2002;121:1921-7. [PubMed]
- Davidson JA, Musk AW, Wood BR, et al. Intralesional cytokine therapy in cancer: a pilot study of GM-CSF infusion in mesothelioma. J Immunother 1998;21:389-98. [PubMed]
- Vandermeers F, Neelature Sriramareddy S, Costa C, et al. The role of epigenetics in malignant pleural mesothelioma. Lung Cancer 2013. [Epub ahead of print]. [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [PubMed]
- Ceresoli GL, Zucali PA, Favaretto AG, et al. Phase II study of pemetrexed plus carboplatin in malignant pleural mesothelioma. J Clin Oncol 2006;24:1443-8. [PubMed]
- Obasaju CK, Ye Z, Wozniak AJ, et al. Single-arm, open label study of pemetrexed plus cisplatin in chemotherapy naive patients with malignant pleural mesothelioma: outcomes of an expanded access program. Lung Cancer 2007;55:187-94. [PubMed]
- Santoro A, O’Brien ME, Stahel RA, et al. Pemetrexed plus cisplatin or pemetrexed plus carboplatin for chemonaive patients with malignant pleural mesothelioma: results of the International Expanded Access Program. J Thorac Oncol 2008;3:756-63. [PubMed]
- Hillerdal G, Sorensen JB, Sundstrom S, et al. Treatment of malignant pleural mesothelioma with carboplatin, liposomized doxorubicin, and gemcitabine: a phase II study. J Thorac Oncol 2008;3:1325-31. [PubMed]
- van Meerbeeck JP, Gaafar R, Manegold C, et al. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol 2005;23:6881-9. [PubMed]
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95. [PubMed]
- Hughes A, Calvert P, Azzabi A, et al. Phase I clinical and pharmacokinetic study of pemetrexed and carboplatin in patients with malignant pleural mesothelioma. J Clin Oncol 2002;20:3533-44. [PubMed]
- Kindler HL, Millard F, Herndon JE 2nd, et al. Gemcitabine for malignant mesothelioma: a phase II trial by the Cancer and Leukemia Group B. Lung Cancer 2001;31:311-7. [PubMed]
- van Meerbeeck JP, Baas P, Debruyne C, et al. A Phase II study of gemcitabine in patients with malignant pleural mesothelioma. European Organization for research and treatment of cancer lung cancer cooperative group. Cancer 1999;85:2577-82. [PubMed]
- Habib EE, Fahmy ES. Chemotherapy management of malignant pleural mesothelioma: a phase II study comparing two popular chemotherapy regimens. Clin Transl Oncol 2013. [Epub ahead of print]. [PubMed]
- Byrne MJ, Davidson JA, Musk AW, et al. Cisplatin and gemcitabine treatment for malignant mesothelioma: a phase II study. J Clin Oncol 1999;17:25-30. [PubMed]
- Nowak AK, Byrne MJ, Williamson R, et al. A multicentre phase II study of cisplatin and gemcitabine for malignant mesothelioma. Br J Cancer 2002;87:491-6. [PubMed]
- Baas P, Ardizzoni A, Grossi F, et al. The activity of raltitrexed (Tomudex) in malignant pleural mesothelioma: an EORTC phase II study (08992). Eur J Cancer 2003;39:353-7. [PubMed]
- Jänne PA, Wozniak AJ, Belani CP, et al. Pemetrexed alone or in combination with cisplatin in previously treated malignant pleural mesothelioma: outcomes from a phase IIIB expanded access program. J Thorac Oncol 2006;1:506-12. [PubMed]
- Jassem J, Ramlau R, Santoro A, et al. Phase III trial of pemetrexed plus best supportive care compared with best supportive care in previously treated patients with advanced malignant pleural mesothelioma. J Clin Oncol 2008;26:1698-704. [PubMed]
- Bearz A, Talamini R, Rossoni G, et al. Re-challenge with pemetrexed in advanced mesothelioma: a multi-institutional experience. BMC Res Notes 2012;5:482. [PubMed]
- Mansour M, Mourad C. Phase II study of single agent oral vinorelbine as first-line treatment in patients with HER-2 negative metastatic breast cancer. Cancer Chemother Pharmacol 2013;72:429-35. [PubMed]
- Caffo O, Dipasquale M, Murgia V, et al. An evaluation of the pharmacokinetics and clinical use of vinorelbine for NSCLC treatment. Expert Opin Drug Metab Toxicol 2013;9:1037-51. [PubMed]
- Steele JP, Shamash J, Evans MT, et al. Phase II study of vinorelbine in patients with malignant pleural mesothelioma. J Clin Oncol 2000;18:3912-7. [PubMed]
- Sørensen JB, Frank H, Palshof T. Cisplatin and vinorelbine first-line chemotherapy in non-resectable malignant pleural mesothelioma. Br J Cancer 2008;99:44-50. [PubMed]
- Stebbing J, Powles T, McPherson K, et al. The efficacy and safety of weekly vinorelbine in relapsed malignant pleural mesothelioma. Lung Cancer 2009;63:94-7. [PubMed]
- Henss H, Fiebig HH, Schildge J, et al. Phase-II study with the combination of cisplatin and doxorubicin in advanced malignant mesothelioma of the pleura. Onkologie 1988;11:118-20. [PubMed]
- Ardizzoni A, Rosso R, Salvati F, et al. Activity of doxorubicin and cisplatin combination chemotherapy in patients with diffuse malignant pleural mesothelioma. An Italian Lung Cancer Task Force (FONICAP) Phase II study. Cancer 1991;67:2984-7. [PubMed]
- Chahinian AP, Antman K, Goutsou M, et al. Randomized phase II trial of cisplatin with mitomycin or doxorubicin for malignant mesothelioma by the Cancer and Leukemia Group B. J Clin Oncol 1993;11:1559-65. [PubMed]
- Arrieta Ó, Medina LA, Estrada-Lobato E, et al. First-line chemotherapy with liposomal doxorubicin plus cisplatin for patients with advanced malignant pleural mesothelioma: phase II trial. Br J Cancer 2012;106:1027-32. [PubMed]
- Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:3007-13. [PubMed]
- Bölükbas S, Manegold C, Eberlein M, et al. Survival after trimodality therapy for malignant pleural mesothelioma: Radical Pleurectomy, chemotherapy with Cisplatin/Pemetrexed and radiotherapy. Lung Cancer 2011;71:75-81. [PubMed]
- Hasani A, Alvarez JM, Wyatt JM, et al. Outcome for patients with malignant pleural mesothelioma referred for Trimodality therapy in Western Australia. J Thorac Oncol 2009;4:1010-6. [PubMed]
- Zucali PA, Ceresoli GL, De Vincenzo F, et al. Advances in the biology of malignant pleural mesothelioma. Cancer Treat Rev 2011;37:543-58. [PubMed]
- Barbieri F, Wurth R, Favoni RE, et al. Receptor tyrosine kinase inhibitors and cytotoxic drugs affect pleural mesothelioma cell proliferation: insight into EGFR and ERK1/2 as antitumor targets. Biochem Pharmacol 2011;82:1467-77. [PubMed]
- Liu Z, Klominek J. Inhibition of proliferation, migration, and matrix metalloprotease production in malignant mesothelioma cells by tyrosine kinase inhibitors. Neoplasia 2004;6:705-12. [PubMed]
- Govindan R, Subramanian J. Optimising therapy for EGFR-addicted NSCLC: just the start. Lancet Oncol 2012;13:216-7. [PubMed]
- Foster JM, Radhakrishna U, Govindarajan V, et al. Clinical implications of novel activating EGFR mutations in malignant peritoneal mesothelioma. World J Surg Oncol 2010;8:88. [PubMed]
- Kindler HL. Systemic treatments for mesothelioma: standard and novel. Curr Treat Options Oncol 2008;9:171-9. [PubMed]
- Kindler HL, Karrison TG, Gandara DR, et al. Multicenter, double-blind, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin plus bevacizumab or placebo in patients with malignant mesothelioma. J Clin Oncol 2012;30:2509-15. [PubMed]
- Gregorc V, Zucali PA, Santoro A, et al. Phase II study of asparagine-glycine-arginine-human tumor necrosis factor alpha, a selective vascular targeting agent, in previously treated patients with malignant pleural mesothelioma. J Clin Oncol 2010;28:2604-11. [PubMed]
- Nowak AK, Brown C, Millward MJ, et al. A phase II clinical trial of the Vascular Disrupting Agent BNC105P as second line chemotherapy for advanced Malignant Pleural Mesothelioma. Lung Cancer 2013. [Epub ahead of print]. [PubMed]
- O’Brien ME, Gaafar RM, Popat S, et al. Phase II study of first-line bortezomib and cisplatin in malignant pleural mesothelioma and prospective validation of progression free survival rate as a primary end-point for mesothelioma clinical trials (European Organisation for Research and Treatment of Cancer 08052). Eur J Cancer 2013;49:2815-22. [PubMed]
- Ceresoli GL, Zucali PA, Mencoboni M, et al. Phase II study of pemetrexed and carboplatin plus bevacizumab as first-line therapy in malignant pleural mesothelioma. Br J Cancer 2013. [Epub ahead of print]. [PubMed]
- Krug LM, Curley T, Schwartz L, et al. Potential role of histone deacetylase inhibitors in mesothelioma: clinical experience with suberoylanilide hydroxamic acid. Clin Lung Cancer 2006;7:257-61. [PubMed]
- Mukohara T, Civiello G, Davis IJ, et al. Inhibition of the met receptor in mesothelioma. Clin Cancer Res 2005;11:8122-30. [PubMed]
- Nasu M, Carbone M, Gaudino G, et al. Ranpirnase Interferes with NF-kappaB Pathway and MMP9 activity, inhibiting malignant mesothelioma cell invasiveness and xenograft growth. Genes Cancer 2011;2:576-84. [PubMed]
- Mikulski SM, Costanzi JJ, Vogelzang NJ, et al. Phase II trial of a single weekly intravenous dose of ranpirnase in patients with unresectable malignant mesothelioma. J Clin Oncol 2002;20:274-81. [PubMed]
- Giovannetti E, Zucali PA, Assaraf YG, et al. Preclinical emergence of vandetanib as a potent antitumour agent in mesothelioma: molecular mechanisms underlying its synergistic interaction with pemetrexed and carboplatin. Br J Cancer 2011;105:1542-53. [PubMed]
- Rischin D, Bibby DC, Chong G, et al. Clinical, pharmacodynamic, and pharmacokinetic evaluation of BNC105P: a phase I trial of a novel vascular disrupting agent and inhibitor of cancer cell proliferation. Clin Cancer Res 2011;17:5152-60. [PubMed]
- Shukla A, Miller JM, Cason C, et al. Extracellular signal-regulated kinase 5: a potential therapeutic target for malignant mesotheliomas. Clin Cancer Res 2013;19:2071-83. [PubMed]
- Zimling ZG, Sorensen JB, Gerds TA, et al. A biomarker profile for predicting efficacy of cisplatin-vinorelbine therapy in malignant pleural mesothelioma. Cancer Chemother Pharmacol 2012;70:743-54. [PubMed]
- Robinson BW, Creaney J, Lake R, et al. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet 2003;362:1612-6. [PubMed]
- Hollevoet K, Reitsma JB, Creaney J, et al. Serum mesothelin for diagnosing malignant pleural mesothelioma: an individual patient data meta-analysis. J Clin Oncol 2012;30:1541-9. [PubMed]
- Grigoriu BD, Chahine B, Vachani A, et al. Kinetics of soluble mesothelin in patients with malignant pleural mesothelioma during treatment. Am J Respir Crit Care Med 2009;179:950-4. [PubMed]
- Hollevoet K, Nackaerts K, Gosselin R, et al. Soluble mesothelin, megakaryocyte potentiating factor, and osteopontin as markers of patient response and outcome in mesothelioma. J Thorac Oncol 2011;6:1930-7. [PubMed]
- Grigoriu BD, Scherpereel A, Devos P, et al. Utility of osteopontin and serum mesothelin in malignant pleural mesothelioma diagnosis and prognosis assessment. Clin Cancer Res 2007;13:2928-35. [PubMed]
- Ting S, Mairinger FD, Hager T, et al. ERCC1, MLH1, MSH2, MSH6, and betaIII-tubulin: resistance proteins associated with response and outcome to platinum-based chemotherapy in malignant pleural mesothelioma. Clin Lung Cancer 2013. [Epub ahead of print]. [PubMed]
- Hirayama N, Tabata C, Tabata R, et al. Pleural effusion VEGF levels as a prognostic factor of malignant pleural mesothelioma. Respir Med 2011;105:137-42. [PubMed]
- Yasumitsu A, Tabata C, Tabata R, et al. Clinical significance of serum vascular endothelial growth factor in malignant pleural mesothelioma. J Thorac Oncol 2010;5:479-83. [PubMed]
- Pinato DJ, Mauri FA, Ramakrishnan R, et al. Inflammation-based prognostic indices in malignant pleural mesothelioma. J Thorac Oncol 2012;7:587-94. [PubMed]
- Kao SC, Pavlakis N, Harvie R, et al. High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res 2010;16:5805-13. [PubMed]
- Kao SC, Klebe S, Henderson DW, et al. Low calretinin expression and high neutrophil-to-lymphocyte ratio are poor prognostic factors in patients with malignant mesothelioma undergoing extrapleural pneumonectomy. J Thorac Oncol 2011;6:1923-9. [PubMed]
- Pinato DJ, Nya P, Sharma R, et al. CD24: a potential new marker in differentiating malignant mesothelioma from pulmonary adenocarcinoma. J Clin Pathol 2013;66:256-9. [PubMed]
- Tabata C, Shibata E, Tabata R, et al. Serum HMGB1 as a prognostic marker for malignant pleural mesothelioma. BMC Cancer 2013;13:205. [PubMed]
- Santarelli L, Strafella E, Staffolani S, et al. Association of MiR-126 with soluble mesothelin-related peptides, a marker for malignant mesothelioma. PloS One 2011;6:e18232. [PubMed]
- Kirschner MB, Cheng YY, Badrian B, et al. Increased circulating miR-625-3p: a potential biomarker for patients with malignant pleural mesothelioma. J Thorac Oncol 2012;7:1184-91. [PubMed]
- Mori T, Tajima K, Hirama M, et al. The N-ERC index is a novel monitoring and prognostic marker for advanced malignant pleural mesothelioma. J Thorac Dis 2013;5:145-8. [PubMed]
- Scherpereel A, Grigoriu B, Conti M, et al. Soluble mesothelin-related peptides in the diagnosis of malignant pleural mesothelioma. Am J Respir Crit Care Med 2006;173:1155-60. [PubMed]
- Sethi S, Ali S, Philip PA, et al. Clinical advances in molecular biomarkers for cancer diagnosis and therapy. Int J Mol Sci 2013;14:14771-84. [PubMed]
- Sturchio E, Amadori A, Businaro J, et al. Possible use of microRNAs as biomarkers for monitoring of workers exposed to asbestos. G Ital Med Lav Ergon 2012;34:571-3. [PubMed]
- Volta V, Ranzato E, Martinotti S, et al. Preclinical demonstration of synergistic Active Nutrients/Drug (AND) combination as a potential treatment for malignant pleural mesothelioma. PloS One 2013;8:e58051. [PubMed]


