Aortic valve replacement in patients with a left ventricular ejection fraction ≤35% performed via a minimally invasive right thoracotomy
Introduction
In patients undergoing cardiac surgery, a reduced preoperative left ventricular ejection fraction is noted in approximately 10% to 15% of patients, and is associated with a worse outcome (1). In those requiring aortic valve replacement (AVR), for either aortic stenosis or aortic regurgitation, a reduced left ventricular ejection fraction is one of the most significant risk factors of early and late mortality (2,3). In addition, clinically significant mitral regurgitation may coexist in up to 35% of this population, which often necessitates a double valve operation (4). However, despite a higher operative mortality, AVR appears to be beneficial in the majority of patients demonstrating better survival coupled with improved ventricular function and functional class (5-7).
Since the inception of minimally invasive valve surgery in the mid-1990s, it has increasingly become accepted as an alternative to conventional median sternotomy (8,9). The benefits of minimally invasive valve surgery include decreased perioperative bleeding, reduced post-operative complications, and faster patient recovery, particularly amongst higher-risk groups (8-10). This includes patients with a reduced left ventricular ejection fraction, those undergoing a double valve operation, and in the setting of re-operative valve surgery (11-17). Therefore, we sought to evaluate the outcomes of patients with aortic valve pathology and a left ventricular ejection fraction of ≤35% who underwent right thoracotomy minimally invasive AVR, with or without concomitant mitral valve (MV) surgery.
Methods
This study was approved by the Mount Sinai Medical Center Institutional Review Board. Our institutional Society of Thoracic Surgeons (STS) Database was retrospectively reviewed to identify patients with severe aortic valve stenosis or regurgitation requiring surgical intervention and a left ventricular ejection fraction of ≤35%, who underwent minimally invasive AVR performed via a right thoracotomy between January 2009 and March 2013, with or without concomitant MV surgery. The variables analyzed included baseline demographics, operative characteristics, perioperative outcomes, and 30-day mortality.
Technique for minimally invasive AVR with or without MV surgery via a right thoracotomy
The surgical approach performed at our institution has been described previously and is briefly summarized here (15,18). A femoral platform was the preferred method to establish cardiopulmonary bypass. Trans-incisional direct aortic cross-clamping was performed in both the aortic and MV procedures. For isolated AVR, a right thoracotomy was performed via a 5 cm to 6 cm right transverse skin incision made approximately 1 cm lateral to the sternum over the second to third intercostal space, and the costochondral cartilage was transected. Debridement and resection of the native aortic valve leaflets was performed with standard techniques, followed by implantation of the aortic prosthesis. For combined aortic and MV surgery, a 6–7 cm incision was performed over the 4th intercostal space starting at the mid-clavicular line and extended laterally, with the MV accessed via a left atriotomy.
MV replacement was performed utilizing a chordal-sparing technique, with excision of the anterior and preservation of the posterior leaflet, or preservation of both leaflets when technically feasible. For MV repair utilizing a ring annuloplasty, the ring was sized according to the surface area or height of the anterior mitral leaflet, with resectional techniques performed as warranted by the underlying pathology. In patients undergoing a transaortic edge-to-edge MV repair, the A2 and P2 segments of the MV were identified after debridement and excision of the native aortic valve. The edge-to-edge repair was performed 1 cm proximal to the leaflet free edges, utilizing a 4–0 Prolene (Ethicon, Somerville, NJ USA) mattress suture reinforced with Teflon (Ethicon) pledgets on the ventricular side of the MV. Patients were candidates for a transaortic edge-to-edge MV repair if the mitral regurgitation jet originated from the A2-P2 leaflet scallops, there was limited leaflet and annular calcification, and there was no significant annular dilatation (19,20).
Statistical methods
All continuous variables were expressed as mean ± 1 standard deviation, or median and interquartile range (IQR, 25% to 75%). Frequencies were expressed as the number and percentage. The Statistical Package for Social Sciences, version 21 (SPSS Inc., Chicago, IL, USA) was used in the data analyses.
Results
There were 75 consecutive patients identified with severe aortic valve stenosis, or regurgitation, with a left ventricular ejection fraction of ≤35% who underwent minimally invasive valve surgery via a right thoracotomy. There were 51 patients who underwent isolated AVR, and 24 patients who had combined AVR and MV surgery for concomitant moderate to severe mitral regurgitation.
Outcomes of isolated AVR
The mean age was 72.1±11.3 years, 7 (13.7%) were females, and 9 (17.6%) had a history of prior cardiac surgery. The mean left ventricular ejection fraction was 28%±6%. Co-morbidities included 21 (41.2%) with a history of prior myocardial infarction, 20 (39.2%) with congestive heart failure, and 10 (19.6%) with chronic obstructive pulmonary disease. The aortic valve pathology was stenosis in 43 (84.3%), regurgitation in 5 (9.8%), and both in 3 (5.9%) patients, respectively (Table 1).
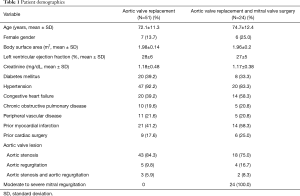
Full table
No patient required conversion to sternotomy, and the median cardiopulmonary bypass time was 116 minutes (IQR, 101–132 minutes). The median total post-operative mechanical ventilation time was 14 hours (IQR, 8–20 hours), and the intensive care unit length of stay was 42 hours (IQR, 26–93 hours). The most common post-operative complication was new-onset atrial fibrillation, which occurred in 15 (29.4%) patients. There were 2 (3.9%) cases of acute kidney injury, and no cerebrovascular accidents. The median hospital length of stay was 7 days (IQR, 5–12 days), and 30-day mortality occurred in 1 (2%) patient (Tables 2,3).
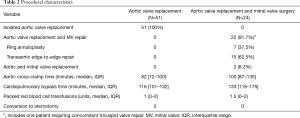
Full table
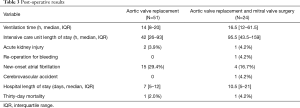
Full table
Outcomes of AVR plus MV surgery
The mean age was 74.7±12.4 years, 6 (25%) were females, and 6 (25%) had a history of prior cardiac surgery. The mean left ventricular ejection fraction was 27%±5%. Co-morbidities included 14 (58.3%) with congestive heart failure, 14 (58.3%) with a history of myocardial infarction, and 5 (20.8%) with peripheral vascular disease. The aortic valve pathology was stenosis in 18 (75.0%), regurgitation in 4 (16.7%), and both in 2 (8.3%) patients, respectively (Table 1).
Concomitant MV repair was performed in 22 (91.7%) patients, and MV replacement was performed in 2 (8.3%). The MV repairs consisted of ring annuloplasty in 7 (37.5%), and a transaortic edge-to-edge repair in 15 (62.5%). Of note, 1 (4.2%) patient who underwent AVR plus MV repair also required tricuspid valve repair for severe tricuspid regurgitation. There were no required conversions to sternotomy, and the median cardiopulmonary bypass time was 133 minutes (IQR, 118–175 minutes) (Table 2). The median total post-operative mechanical ventilation time was 16.5 hours (IQR, 12–61.5 hours), and the intensive care unit length of stay was 95.5 hours (IQR, 43.5–159 hours). The most common postoperative complication was new-onset atrial fibrillation, which occurred in 4 (16.7%) patients. There was 1 (4.2%) case of acute kidney injury, and 1 (4.2%) cerebrovascular accident. The median hospital length of stay was 10.5 days (IQR, 5–21 days), and 30-day mortality occurred in 1 (4.2%) patient (Table 3).
Discussion
Patients undergoing AVR with a reduced left ventricular systolic function are at a substantial risk for perioperative morbidity and mortality, and account for approximately 11.5–13% of patients undergoing AVR (6). The operative mortality is also increased in those undergoing double valve procedures, being 9.4% to 10.7% for those undergoing concomitant aortic and MV surgery (21,22). Higher-risk patients are far less likely to undergo AVR based on their clinical risk profiles, with a reduced left ventricular ejection fraction decreasing the likelihood of intervention by more than 2-fold (23,24). This is in spite of the established benefit of AVR in terms of improvement in systolic function and reverse remodeling of the left ventricle (25,26). As such, data suggests that a minimally invasive approach, with its enhanced recovery, may be a viable alternative in this high-risk population (9,10). Indeed, the present study demonstrated excellent outcomes, with a fairly low incidence in morbidity, reasonable hospital lengths of stay, and a thirty-day morality of 2% and 4.2% in patients with a left ventricular ejection fraction ≤35% who underwent isolated AVR or AVR plus MV surgery, respectively.
The data on minimally invasive surgery in patients with aortic valve pathology and reduced left ventricular ejection fraction are limited. Tabata et al., conducted a retrospective review of 140 patients with a left ventricular ejection fraction ≤40% who underwent isolated AVR via an upper hemi-sternotomy (11). Two matched cohorts of 41 patients each were constructed using a propensity score analysis to compare the surgical approaches. The incidence of operative mortality (2.4% vs. 4.8%, P=0.56), acute kidney injury (0% vs. 2.4%, P=0.32), cerebrovascular accidents (0% vs. 2.4%, P=0.32), and blood transfusion requirement (46.3% vs. 31.7%, P=0.17) were similar between the minimally invasive and median sternotomy groups. Also noted were similar hospital lengths of stay (8.5 vs. 10.6 days, P=0.17) and discharge to home rates (73.2% vs. 56.1%, P=0.13). Subsequently Nguyen et al., retrospectively evaluated 688 patients who underwent AVR via a right thoracotomy, and 815 who had AVR via a standard median sternotomy (12). A propensity analysis was performed to compare 35 matched pairs of patients undergoing right thoracotomy versus median sternotomy AVR in the setting of a left ventricular ejection fraction ≤40%. There was no difference in the intensive care unit length of stay (69 vs. 72.6 h, P=0.80), postoperative hospital length of stay (10.3 vs. 7.2 days, P=0.13), or 30-day mortality (3.8% vs. 0.8%, P=0.50). The results of these two studies demonstrate that a minimally invasive approach to AVR in the setting of left ventricular systolic dysfunction can be performed safely, with morbidity and mortality outcomes similar to full sternotomy. It should be noted, however, that the small number of patients included limits the statistical power to elucidate differences in clinical outcomes, and the results should be interpreted within this context.
In the 2017 update to the valvular heart disease guidelines, the American College of Cardiology/American Heart Association have assigned a class IA recommendation for transcatheter AVR (TAVR) as an alternative to surgical AVR in high-risk patients, given at least comparable outcomes between the approaches in randomized controlled trials (27-29). However, direct comparisons between a minimally invasive approach to AVR and TAVR are scarce. Miceli et al., presented the outcomes of 74 propensity-matched patients who underwent minimally invasive sutureless AVR via a right thoracotomy or TAVR (30). The mean age of the cohort was 79±6 years, with a median logistic EuroSCORE of 14% (range 9–20%). While there was a higher incidence of paravalvular leak in the TAVR group, there were no differences in in-hospital mortality (8.1% vs. 0%, P=0.25), cerebrovascular accident (5.2% vs. 0%, P=0.3), or 2-year survival, (66.2% vs. 91.2%, P=0.1), when compared with minimally invasive AVR. Future prospective studies are needed to assess the optimal approach to less invasive AVR in high-risk populations.
In the present study, 24 (32%) patients required double valve surgery for aortic valve disease and concomitant moderate to severe mitral regurgitation, which increases the risk and complexity of the operation. Despite the severely reduced left ventricular systolic function and clinical risk profile, a right thoracotomy approach resulted in acceptable perioperative outcomes and a low 30-day mortality, with an excellent MV repair rate of 91.7%. Approximately two-thirds of the patients underwent a transaortic edge-to-edge MV repair, which avoids extensive mediastinal dissection and the need for an atriotomy, and requires approximately 10 additional minutes in operative time (19,20,31,32). Thus, the decreased surgical trauma afforded by this technique may be of particular benefit in high-risk patients (33). Strict selection criteria based on preserved annular and leaflet mechanics and anatomy as described herein, is of great importance to reduce the risk of recurrent mitral regurgitation given the lack of a ring annuloplasty, and to avoid the possibility of functional mitral stenosis (34,35).
The present study is subject to the limitations found in a single center, retrospective study design, which is associated with an inherent selection bias. In both groups of patients the aortic valve pathology varied, which can have important therapeutic and operative implications as aortic stenosis and regurgitation each impart different hemodynamic loads and remodeling changes upon the ventricle. In patients who underwent concomitant MV surgery, the etiology of the mitral regurgitation is unknown. This is important as significant secondary mitral regurgitation may improve in up to 50% of patients undergoing AVR for aortic stenosis, due to favorable changes in the left ventricular hemodynamic loading and reverse remodeling that occurs post-operatively (5,36). Finally, there is no median sternotomy comparison group, the clinical follow-up time period is limited to 30 days, and serial echocardiographic examination was not available, limiting the conclusions that may be inferred from the present data.
In conclusion, in patients with severe aortic stenosis or regurgitation in the setting of a left ventricular ejection fraction of ≤35%, minimally invasive AVR via a right thoracotomy can be performed safely and effectively for isolated aortic valve disease or in combination with MV surgery, with a low morbidity and mortality. It may be considered as an alternative to median sternotomy, with future studies comparing this approach to TAVR needed to define the optimal less invasive approach to high-risk AVR.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was approved by the Institutional Review Board at the Mount Sinai Medical Center, Miami Beach, Florida, USA.
References
- Pieri M, Belletti A, Monaco F, et al. Outcome of cardiac surgery in patients with low preoperative ejection fraction. BMC Anesthesiol 2016;16:97. [Crossref] [PubMed]
- Hannan EL, Samadashvili Z, Lahey SJ, et al. Aortic valve replacement for patients with severe aortic stenosis: risk factors and their impact on 30-month mortality. Ann Thorac Surg 2009;87:1741-49. [Crossref] [PubMed]
- Chaliki HP, Mohty D, Avierinos JF, et al. Outcomes after aortic valve replacement in patients with severe aortic regurgitation and markedly reduced left ventricular function. Circulation 2002;106:2687-93. [Crossref] [PubMed]
- Nombela-Franco L, Ribeiro HB, Urena M, et al. Significant mitral regurgitation left untreated at the time of aortic valve replacement: a comprehensive review of a frequent entity in the transcatheter aortic valve replacement era. J Am Coll Cardiol 2014;63:2643-58. [Crossref] [PubMed]
- Vaquette B, Corbineau H, Laurent M, et al. Valve replacement in patients with critical aortic stenosis and depressed left ventricular function: predictors of operative risk, left ventricular function recovery, and long term outcome. Heart 2005;91:1324-29. [Crossref] [PubMed]
- Flores-Marín A, Gómez-Doblas JJ, Caballero-Borrego J, et al. Long-term predictors of mortality and functional recovery after aortic valve replacement for severe aortic stenosis with left ventricular dysfunction. Rev Esp Cardiol 2010;63:36-45. [PubMed]
- Bhudia SK, McCarthy PM, Kumpati GS, et al. Improved outcomes after aortic valve surgery for chronic aortic regurgitation with severe left ventricular dysfunction. J Am Coll Cardiol 2007;49:1465-71. [Crossref] [PubMed]
- Navia JL, Cosgrove DM 3rd. Minimally invasive mitral valve operations. Ann Thorac Surg 1996;62:1542-44. [Crossref] [PubMed]
- Schmitto JD, Mokashi SA, Cohn LH. Minimally-invasive valve surgery. J Am Coll Cardiol 2010;56:455-62. [Crossref] [PubMed]
- Santana O, Xydas S, Williams RF, et al. Minimally invasive valve surgery in high-risk patients. J Thorac Dis 2017. [Epub ahead of print].
- Tabata M, Aranki SF, Fox JA, et al. Minimally invasive aortic valve replacement in left ventricular dysfunction. Asian Cardiovasc Thorac Ann 2007;15:225-28. [Crossref] [PubMed]
- Nguyen TC, Thourani VH, Pham JQ, et al. Traditional Sternotomy Versus Minimally Invasive Aortic Valve Replacement in Patients Stratified by Ejection Fraction. Innovations (Phila) 2017;12:33-40. [Crossref] [PubMed]
- Santana O, Reyna J, Pineda AM, et al. Outcomes of minimally invasive mitral valve surgery in patients with an ejection fraction of 35% or less. Innovations (Phila) 2013;8:1-5. [Crossref] [PubMed]
- Atik FA, Svensson LG, Blackstone EH, et al. Less invasive versus conventional double-valve surgery: a propensity-matched comparison. J Thorac Cardiovasc Surg 2011;141:1461-8.e4. [Crossref] [PubMed]
- Pineda AM, Santana O, Reyna J, et al. Outcomes of reoperative aortic valve replacement via right mini-thoracotomy versus median sternotomy. J Heart Valve Dis 2013;22:50-55. [PubMed]
- Mihos CG, Capoulade R, Yucel E, et al. Combined papillary muscle sling and ring annuloplasty for moderate- to-severe secondary mitral regurgitation. J Card Surg 2016;31:664-71. [Crossref] [PubMed]
- Santana O, Reyna J, Benjo AM, et al. Outcomes of minimally invasive valve surgery in patients with chronic obstructive pulmonary disease. Eur J Cardiothorac Surg 2012;42:648-52. [Crossref] [PubMed]
- Santana O, Reyna J, Grana R, et al. Outcomes of minimally invasive valve surgery versus standard sternotomy in obese patients undergoing isolated valve surgery. Ann Thorac Surg 2011;91:406-10. [Crossref] [PubMed]
- Mihos CG, Santana O, Brenes JC, et al. Outcomes of transaortic edge-to-edge repair of the mitral valve in patients undergoing minimally invasive aortic valve replacement. J Thorac Cardiovasc Surg 2013;145:1412-13. [Crossref] [PubMed]
- Santana O, Lamelas J. Minimally invasive transaortic repair of the mitral valve. Heart Surg Forum 2011;14:E232-36. [Crossref] [PubMed]
- Vassileva CM, Li S, Thourani VH, et al. Outcome characteristics of multiple-valve surgery: comparison with single-valve procedures. Innovations (Phila) 2014;9:27-32. [Crossref] [PubMed]
- Rankin JS, He X, O’Brien SM, et al. The Society of Thoracic Surgeons risk model for operative mortality after multiple valve surgery. Ann Thorac Surg 2013;95:1484-90. [Crossref] [PubMed]
- Bach DS, Siao D, Girard SE, et al. Evaluation of patients with severe symptomatic aortic stenosis who do not undergo aortic valve replacement: the potential role of subjectively overestimated operative risk. Circ Cardiovasc Qual Outcomes 2009;2:533-39. [Crossref] [PubMed]
- Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 2005;26:2714-20. [Crossref] [PubMed]
- Badar AA, Brunton AP, Mahmood AH, et al. The management of patients with aortic regurgitation and severe left ventricular dysfunction: a systematic review. Expert Rev Cardiovasc Ther 2015;13:915-22. [Crossref] [PubMed]
- Sharma UC, Barenbrug P, Pokharel S, et al. Systematic review of the outcome of aortic valve replacement in patients with aortic stenosis. Ann Thorac Surg 2004;78:90-95. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017.2017. [Epub ahead of print]. [PubMed]
- Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2477-84. [Crossref] [PubMed]
- Deeb GM, Reardon MJ, Chetcuti S, et al. 3-year outcomes in high-risk patients who underwent surgical or transcatheter aortic valve replacement. J Am Coll Cardiol 2016;67:2565-74. [Crossref] [PubMed]
- Miceli A, Gilmanov D, Murzi M, et al. Minimally invasive aortic valve replacement with a sutureless valve through a right anterior mini-thoracotomy versus transcatheter aortic valve implantation in high-risk patients. Eur J Cardiothorac Surg 2016;49:960-65. [Crossref] [PubMed]
- Källner G, van der Linden J, Hadjinikolaou L, et al. Transaortic approach for the Alfieri stitch. Ann Thorac Surg 2001;71:378-79. [Crossref] [PubMed]
- Kavarana MN, Edwards NM, Levinson MM, et al. Transaortic repair of mitral regurgitation. Heart Surg Forum 2000;3:24-28. [PubMed]
- Mihos CG, Larrauri-Reyes M, Hung J, et al. Transaortic Edge-to-Edge Repair for Functional Mitral Regurgitation During Aortic Valve Replacement: A 13-Year Experience. Innovations (Phila) 2016;11:425-9. [Crossref] [PubMed]
- De Bonis M, Lapenna E, Pozzoli A, et al. Edge-to-edge surgical mitral valve repair in the era of MitraClip: what if the annuloplasty ring is missed? Curr Opin Cardiol 2015;30:155-60. [Crossref] [PubMed]
- Maisano F, Viganò G, Blasio A, et al. Surgical isolated edge-to-edge mitral valve repair without annuloplasty: clinical proof of the principle for an endovascular approach. EuroIntervention 2006;2:181-86. [PubMed]
- Wyler S, Emmert MY, Biaggi P, et al. What happens to functional mitral regurgitation after aortic valve replacement for aortic stenosis? Heart Surg Forum 2013;16:E238-42. [Crossref] [PubMed]


