Ventricular assist devices: initial orientation
Introduction
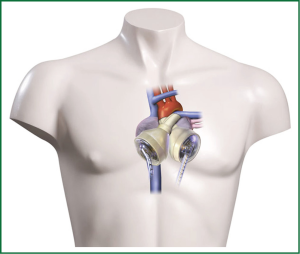
Heart transplantation (HTx) as the ‘golden standard’ for patients suffering from end stage heart failure, becomes only for a small patient population a true therapeutic option. Recipients have to be well selected as there are contraindications like high pulmonary resistance, malignant tumors, persisting infections or age. Further the permanent organ shortage worsens the situation for recipients (1). Development of alternative techniques like ventricular assist devices (VAD) or total artificial heart (TAH, Figure 1) have evolved over the last years. Since the REMATCH trial reported the beneficial effects of long-term mechanical left ventricular support with 52% one year survival compared to 25% (P=0.02) optimized medical treatment (2) the evolution from large pulsatile-flow VAD technology to continuous-flow (cf) resulted in substantially increased survival rates. Recently the fifth report of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) was published analysing over 6,000 patients provided with a mechanical circulatory support system (MCS) reporting actuarial survival of 80% for 1 year and 70% for 2 years. Today the primary goal of MCS, keeping the patient alive and bridge to HTx (Bridge to transplantation, BTT), has been added with acceptable quality of life (QoL) and permanent support (Bridge to destination, BTD). Recent systems have only a fraction of the weight of first generation VADs and offer the patient a higher flexibility with regard to battery capacity. Due to this development numbers of implantable left VADs (LVADs) especially as BTD is increasing strongly (3).
Purpose of MCS
In the beginning the first effort of MCS was to support the heart if weaning from bypass was not possible (4) for a short period of time as a bridge to recovery (BTR). However when myocardial recovery was not achieved HTx remained the only option. While waiting times for a suitable donor heart increased MCS support time emerged from short to mid-term. Similar for patients deteriorating while waiting for HTx, MCS became an option (BTT). As it was reported that VAD therapy had superior results in patients suffering from terminal heart failure waiting for HTx is superior compared with inotropic support (2) or compared to medical treatment in non-transplant candidates (5) implantation numbers rose drastically. Finally due to advantages in technology, the worsening donor shortage, increased life expectancy and rising numbers of heart failure patients’ permanent support became realistic (BTD).
Across the years gaining more experiences with VAD treatment, in a number of patients sustained myocardial recovery was noticed resulting in weaning from VAD (6-9). Today percentage of BTT decreases, while BTR is stable but with a low percentage, and the rapid growing filed of permanent support. Gaining more attention is the concept of bridging to decision or candidacy in pateinst in whom a contraindication for HTx exist but may be reversal (i.e., curative treated malignant tumor, pulmonary hypertension) (10).
VAD
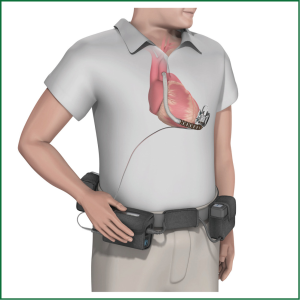
Implantation of different types of VAD is now an established treatment both for patients with chronic end-stage heart failure of different origins and those with acute heart failure. It has to differentiate between TAH replacing the heart like the Cardiowest System (see Figure 1; see below) or VAD supporting either the left ventricle (LVAD), the right ventricle (RVAD) or both ventricles (BVAD). The percentage of biventricular support has constantly dropped over the last years to less than 25% (11). Today small implantable LVADs of the second (as example see Figure 2) and third (as example see Figure 3) generation are working with a cf, are small, fast and easy to implant and have a better reliability compared to the old pulsatile flow devices. So far, no severe negative impact of the ‘non-physiologic’ flow, has been reported (12-14). CF-LVADs demonstrate a low incidence of bleeding and infection and offer better QoL, especially if long-term support is anticipated compared to BVAD. Implantable LVADs are now broadly employed worldwide with growing acceptance. Using different new generation cf-LVADs studies demonstrated up to 91% six month and 86% one year survival (15-19). Nevertheless currently no available devices fulfill ‘optimal’ requirements comprising durability without technical failure, a non-thrombogenic blood contact surface and a fully implantable with transcutaneous energy transfer and an implantable control unit, without leads penetrating the patient’s skin.

TAHs
In some clinical conditions like large myocardial acute infarction, large intraventricular thrombus formation or cardiac tumors, replacement of the whole failing heart using a TAH may become necessary. The Cardiowest (SynCardia, Tucson, AZ, USA) is an approved TAH by the Food and Drug administration for the clinical. Briefly, it is pneumatically driven, consisting of prosthetic ventricles made out of polyurethane, and has mechanical valves. Surgical it is attached to the left and right atria. In a large US multicentre trial using the TAH one year survival of 79% was observed (20). Recently Leprince and colleagues reported a 74% 30-day survival in their ten year experience (21).
Patient selection
Patient selection and timing remain crucial factors for improving outcomes in VAD recipients. Patients presenting on inotropic dependent end-stage heart failure with worsening clinical conditions (critical peripheral perfusion, metabolic acidosis; cardiac index <2.0 L/m2/min, mixed venous oxygen saturation <40%, early signs of renal, hepatic or multi-organ failure without surgical options) should be considered for MCS. Simple forms of MCS therapy (e.g., IABP) may be not sufficient to increase cardiac output. If a fast recovery can be expected extracorporeal membrane oxygenation (ECMO) will be sufficient; but the use of ECMO is limited by days to weeks. Therefore besides a few contraindications like ongoing malignant neoplastic diseases with a very limited life expectancy, refusal by the patient or advanced multi-organ failure a VAD implant should be considered. There is evidence that making decision in favor of earlier VAD implantation results in better outcome (22). One important determinante is the function of the right ventricle (RV). Numerous published scores and single parameters are regarded as helpful (23-25); Nevertheless echocardiography remains the most reliable: RV dimensions, degree of tricuspid regurgitation (TR), right ventricular ejection fraction, right atrial diameter as well as the pulmonary resistance (26). Earlier VAD placement tends to avoid RV failure and subsequent BVAD implantation. Outcome even of implantable BVAD is inferior compared to LVADs (27). Nevertheless when biventricular failure has already occurred either BVAD or TAH has to be implanted to save the patient’s life.
Surgical considerations in LVADs
Besides the pre-operative status of the patient and RV function, surgical access [median sternotomy vs. left lateral thoracotomy (LLT)], optimal positioning of the inflow cannula (directed posteriorly toward the mitral valve), valve pathologies, and exit side of the percutaneous lead have to be considered. Most of the implantations are done using median sternotomy. Then the outflow graft of the LVAD is anastomosed to the ascending part of the aorta. Alternative a LLT using the fifth intercostal space is another technique (28). In patients with previous cardiac operations this will avoid repeat sternotomy reducing the risk of bleeding or harming a mammary bypass (29). In this case the outflow tract of the LVAD may be sutured to the descending aorta. Depending on the dimension of an implantable LVAD, surgical creation of a ‘pump pocket’ may become necessary. Whereas for the pulsatile -flow LVADs large preperitoneal or even intraperitoneal pump pockets had to be created (30); Today small cf-LVADs may only need a small preperitoneal pump pocket (left rectus muscle, above the posterior rectus sheath) (31) or some designs allows the device to fit within the pericardial space (32). For diagnostic reasons intraoperative echocardiography is essential to confirm correct position inflow cannula, identifying valvular pathology, intracardiac thrombi, or a patent foramen ovale (PFO). Especially attention must be paid to the aortic valve: pre-existing aortic mechanical valves should be either replaced by a tissue valve or the outflow tract over-sewn to avoid thromboembolic events (33). Aortic valve regurgitation tends to have a significant effect on pump performance and the estimated flow rates. Therefore aortic valve replacement by a biological prosthesis is recommended (33). If severe aortic insufficiency occurs post-LVAD placement, percutaneous transcatheter aortic valve closure evolves as an alternative to re-sternotomy (34). Aortic stenosis is generally insignificant with regard to pump performance and does not need to be corrected. Contrary mitral valve stenosis needs to be fixed to optimize ventricular filling whereas mitral insufficiency does not require repair (31). Surgical correction of severe TR at the time of implantation confers better long-term outcome as it improves early right-heart function (35). A persistent PFO has to be closed at the time of operation to avoid hemodynamic impairment (36). Placement and externalization of the drive line is of importance to avoid infection or damage to the driveline. The percutaneous lead exit site location should be marked before the surgical procedure and may be located either in the right or left lower quadrant of the abdominal wall about 4 fingers above the spina iliaca anterior superior. The lead will be percutaneously tunnelled exiting the pump housing with a gentle curve (31).
Anticoagulation
Anti-coagulation therapy remains an essential part during support with VAD to avoid thrombotic complications (37). Diverse regimes have been introduced for different types of VAD; Nevertheless thromboembolic complication remains a major adverse event throughout time of VAD support (38). To achieve a balanced anticoagulation coagulation laboratory including the international normalized ratio (INR), thrombocyte aggregation, and thrombelastography is necessary. Most anticoagulation protocols include combinations of coumadin or warfarin and antiplatelet therapy (acetylsalicylic, clopidogrel, dipyridamol). Postoperatively, after achieving adequate hemostasis, use of i.v. heparin will be administered as transition to long-term oral anticoagulation (i.e., warfarin) therapy (31). Depending on the type of LVAD INR levels may be as low as 1.5 (Heartmate II) (39) up to 3.5 (for the Berlin Heart Excor) (40). In situations with increased endothelial activation and platelet aggregation like infections/bacteremia anticoagulation has to be adjusted to the clinical condition. Acquired von Willebrandts disease may occur in patients supported with cf LVADs associated with GI bleeding and other bleeding events requiring reduction of anti-coagulation (31,41).
Pediatric VAD
Contrarily to adults VADs for all age groups of children are still in their infancy. Several adult designed VADs have been adopted for the use in children like the Heartmate II used in teenager between 12 and 13 years (42) or the Heartware HVAD implanted in children reported as young as 6 years (43). Likewise the HeartAssist 5 Pediatric VAD (MicroMed Technology Inc, Houston, TX) former known as DeBakey VAD Child may be used for children older than 5 years. Nevertheless for the smallest only two extracorporeal pneumatically driven devices are commercially available (Medos HIA and the Berlin Heart Excor). In children up to 0.7 m2 body surface area the Berlin Heart Excor pediatric VAD [Berlin Heart AG, Berlin, Germany) remains the only VAD for long-term support. Even in neonates mechanical support using the Berlin Heart Excor achieved a survival of 70% (44). Recent studies revealed that complication rates in children differ from adults (45,46)]; The US investigational device exemption (IDE) multi-centre trial reported a serious adverse events, including infection, stroke and bleeding, with 0.07 events per patient-day in the VAD group and with 0.08 events per patient-day in the ECMO group (47). Besides the limited availability of VAD in children core issues of further research are anticoagulation regimen minimizing thromboembolic events and bleeding complications as so far No standard anticoagulation protocol has been developed and this might be even more important in adult sized VAD used in children as pump speed might be lower compared to adults.
Conclusions
Implantation of different types of LVADs is now broadly employed worldwide and numbers are steadily increasing. Better QoL of implanted LVAD compared to BVAD may lead to earlier device implantation; especially as LVAD safety profile, clinical management and outcomes continue to improve with acceptable complications rates. Challenges remain; the appropriate amount of anticoagulation, avoiding driveline infection and its management and finally results of true ‘long-term’ support has to be observed.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Schweiger M, Wasler A, Prenner G, et al. Improving the rate of organ donation. Transplant Proc 2004;36:2543-5. [PubMed]
- Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435-43. [PubMed]
- Krabatsch T, Schweiger M, Stepanenko A, et al. Improvements in implantable mechanical circulatory support systems: literature overview and update. Herz 2011;36:622-9. [PubMed]
- Liotta D, Hall CW, Henly WS, et al. Prolonged assisted circulation during and after cardiac or aortic surgery. Prolonged partial left ventricular bypass by means of intracorporeal circulation. Am J Cardiol 1963;12:399-405. [PubMed]
- Aaronson KD, Eppinger MJ, Dyke DB, et al. Left ventricular assist device therapy improves utilization of donor hearts. J Am Coll Cardiol 2002;39:1247-54. [PubMed]
- Birks EJ. Myocardial recovery in patients with chronic heart failure: is it real? J Card Surg 2010;25:472-7. [PubMed]
- Dandel M, Weng Y, Siniawski H, et al. Long-term results in patients with idiopathic dilated cardiomyopathy after weaning from left ventricular assist devices. Circulation 2005;112:I37-45. [PubMed]
- Krabatsch T, Schweiger M, Dandel M, et al. Is bridge to recovery more likely with pulsatile left ventricular assist devices than with nonpulsatile-flow systems? Ann Thorac Surg 2011;91:1335-40. [PubMed]
- Potapov EV, Schweiger M, Krabatsch T. Percutaneous balloon occlusion of a left ventricular assist device outflow cannula to facilitate evaluation of myocardial recovery. J Heart Lung Transplant 2011;30:1300-1. [PubMed]
- Potapov EV, Weng Y, Jurmann M, et al. Bridging to transplantability with a ventricular assist device. J Thorac Cardiovasc Surg 2005;130:930. [PubMed]
- Kirklin JK, Naftel DC, Kormos RL, et al. The Fourth INTERMACS Annual Report: 4,000 implants and counting. J Heart Lung Transplant 2012;31:117-26. [PubMed]
- Kamdar F, Boyle A, Liao K, et al. Effects of centrifugal, axial, and pulsatile left ventricular assist device support on end-organ function in heart failure patients. J Heart Lung Transplant 2009;28:352-9. [PubMed]
- Radovancevic B, Vrtovec B, de Kort E, et al. End-organ function in patients on long-term circulatory support with continuous- or pulsatile-flow assist devices. J Heart Lung Transplant 2007;26:815-8. [PubMed]
- Slaughter MS, Tsui SS, El-Banayosy A, et al. Results of a multicenter clinical trial with the Thoratec Implantable Ventricular Assist Device. J Thorac Cardiovasc Surg 2007;133:1573-80. [PubMed]
- Wieselthaler GM, O Driscoll G, Jansz P, et al. Initial clinical experience with a novel left ventricular assist device with a magnetically levitated rotor in a multi-institutional trial. J Heart Lung Transplant 2010;29:1218-25. [PubMed]
- John R, Kamdar F, Liao K, et al. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg 2008;86:1227-34; discussion 1234-5. [PubMed]
- Lahpor J, Khaghani A, Hetzer R, et al. European results with a continuous-flow ventricular assist device for advanced heart-failure patients. Eur J Cardiothorac Surg 2010;37:357-61. [PubMed]
- Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241-51. [PubMed]
- Kirklin JK, Naftel DC, Kormos RL, et al. Third INTERMACS Annual Report: the evolution of destination therapy in the United States. J Heart Lung Transplant 2011;30:115-23. [PubMed]
- Copeland JG, Smith RG, Arabia FA, et al. Cardiac replacement with a total artificial heart as a bridge to transplantation. N Engl J Med 2004;351:859-67. [PubMed]
- Kirsch ME, Nguyen A, Mastroianni C, et al. SynCardia temporary total artificial heart as bridge to transplantation: current results at la pitié hospital. Ann Thorac Surg 2013;95:1640-6. [PubMed]
- Potapov EV, Loforte A, Weng Y, et al. Experience with over 1000 implanted ventricular assist devices. J Card Surg 2008;23:185-94. [PubMed]
- Matthews JC, Koelling TM, Pagani FD, et al. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol 2008;51:2163-72. [PubMed]
- Fitzpatrick JR 3rd, Frederick JR, Hsu VM, et al. Risk score derived from pre-operative data analysis predicts the need for biventricular mechanical circulatory support. J Heart Lung Transplant 2008;27:1286-92. [PubMed]
- Drakos SG, Janicki L, Horne BD, et al. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am J Cardiol 2010;105:1030-5. [PubMed]
- Potapov EV, Stepanenko A, Dandel M, et al. Tricuspid incompetence and geometry of the right ventricle as predictors of right ventricular function after implantation of a left ventricular assist device. J Heart Lung Transplant 2008;27:1275-81. [PubMed]
- Krabatsch T, Potapov E, Stepanenko A, et al. Biventricular circulatory support with two miniaturized implantable assist devices. Circulation 2011;124:S179-86. [PubMed]
- Pasic M, Bergs P, Hennig E, et al. Simplified technique for implantation of a left ventricular assist system after previous cardiac operations. Ann Thorac Surg 1999;67:562-4. [PubMed]
- Frazier OH, Rose EA, McCarthy P, et al. Improved mortality and rehabilitation of transplant candidates treated with a long-term implantable left ventricular assist system. Ann Surg 1995;222:327-36; discussion 336-8. [PubMed]
- Wasler A, Springer WE, Radovancevic B, et al. A comparison between intraperitoneal and extraperitoneal left ventricular assist system placement. ASAIO J 1996;42:M573-6. [PubMed]
- Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant 2010;29:S1-39. [PubMed]
- Gregoric ID, Cohn WE, Frazier OH. Diaphragmatic implantation of the HeartWare ventricular assist device. J Heart Lung Transplant 2011;30:467-70. [PubMed]
- Dranishnikov N, Stepanenko A, Potapov EV, et al. Simultaneous aortic valve replacement in left ventricular assist device recipients: single-center experience. Int J Artif Organs 2012;35:489-94. [PubMed]
- Parikh KS, Mehrotra AK, Russo MJ, et al. Percutaneous transcatheter aortic valve closure successfully treats left ventricular assist device-associated aortic insufficiency and improves cardiac hemodynamics. JACC Cardiovasc Interv 2013;6:84-9. [PubMed]
- Deo SV, Hasin T, Altarabsheh SE, et al. Concomitant tricuspid valve repair or replacement during left ventricular assist device implant demonstrates comparable outcomes in the long term. J Card Surg 2012;27:760-6. [PubMed]
- Krabatsch T, Stepanenko A, Schweiger M, et al. Alternative technique for implantation of biventricular support with HeartWare implantable continuous flow pump. ASAIO J 2011;57:333-5. [PubMed]
- Rossi M, Serraino GF, Jiritano F, et al. What is the optimal anticoagulation in patients with a left ventricular assist device? Interact Cardiovasc Thorac Surg 2012;15:733-40. [PubMed]
- John R, Kamdar F, Liao K, et al. Low thromboembolic risk for patients with the Heartmate II left ventricular assist device. J Thorac Cardiovasc Surg 2008;136:1318-23. [PubMed]
- Boyle AJ, Russell SD, Teuteberg JJ, et al. Low thromboembolism and pump thrombosis with the HeartMate II left ventricular assist device: analysis of outpatient anti-coagulation. J Heart Lung Transplant 2009;28:881-7. [PubMed]
- Krabatsch T, Schweiger M, Stepanenko A, et al. Mechanical circulatory support-results, developments and trends. J Cardiovasc Transl Res 2011;4:332-9. [PubMed]
- Geisen U, Heilmann C, Beyersdorf F, et al. Non-surgical bleeding in patients with ventricular assist devices could be explained by acquired von Willebrand disease. Eur J Cardiothorac Surg 2008;33:679-84. [PubMed]
- Owens WR, Bryant R 3rd, Dreyer WJ, et al. Initial clinical experience with the HeartMate II ventricular assist system in a pediatric institution. Artif Organs 2010;34:600-3. [PubMed]
- Miera O, Potapov EV, Redlin M, et al. First experiences with the HeartWare ventricular assist system in children. Ann Thorac Surg 2011;91:1256-60. [PubMed]
- Stiller B, Weng Y, Hübler M, et al. Pneumatic pulsatile ventricular assist devices in children under 1 year of age. Eur J Cardiothorac Surg 2005;28:234-9. [PubMed]
- Morales DL, Almond CS, Jaquiss RD, et al. Bridging children of all sizes to cardiac transplantation: the initial multicenter North American experience with the Berlin Heart EXCOR ventricular assist device. J Heart Lung Transplant 2011;30:1-8. [PubMed]
- Schweiger M, Schrempf J, Sereinigg M, et al. Complication profile of the berlin heart excor biventricular support in children. Artif Organs 2013;37:730-5. [PubMed]
- Fraser CD Jr, Jaquiss RD, Rosenthal DN, et al. Prospective trial of a pediatric ventricular assist device. N Engl J Med 2012;367:532-41. [PubMed]


