Which type of surgery should become the preferred procedure for malignant pleural mesothelioma: extrapleural pneumonectomy or extended pleurectomy?
Introduction
Malignant pleural mesothelioma (MPM) is a thoracic tumor with high mortality rates. Patients frequently present with dyspnea, chest pain, and effusion. MPM is often caused by exposure to asbestos, which is one of the etiological factors in a large proportion of patients in Turkey.
MPM cases are difficult for thoracic surgeons to diagnose or to specify a possible prognosis, as they exhibit symptoms similar to several other medical conditions as we can provide a prognostic estimate only within an operative observation framework after obtaining information from frozen section after surgery of lung cancer cases. While cytoreductive surgery in the early stage provides a good prognostic factor, the general consensus among surgeons is that there are unknown prognostic markers. These characteristics make MPM more difficult to detect and manage, making its treatment one of the most challenging in cases of tumors that can be managed by thoracic surgery.
Currently, the importance of multimodal therapy is an accepted practice in MPM treatment, as is the aggressive surgical option of extrapleural pneumonectomy (EPP) first used in the 1970s. A recently published study on mesothelioma and radical surgery (MARS) discusses the role of radical surgery in MPM (1). The MARS study reports some hopeful results from therapies such as immunotherapy and gene therapy, motivating fresh perspectives in the treatment of MPM, besides alternative applications in multimodal therapy (2,3).
In our study, which is a single-center retrospective study, we analyze the results of MPM cases, provide an overview of the long-term results and effectiveness of EPP, and compare EPP with other surgical options, with the aim of achieving better results in the treatment of MPM.
Patients and methods
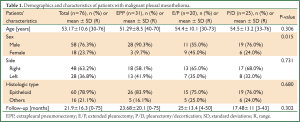
This study involves 76 consecutive patients treated in a single institution between January 2001 and January 2013, and is based on retrospective clinical data. There were 58 males (76%) and 18 females (24%), with a median age of 53.17±10.93 years (30-76 years). MPM was located on the right side in 48 patients (63%) and on the left side in 28 patients (37%). Occupational asbestosis was found in 48 patients (63%) whom spent a part of their lives in places where asbestosis was endemic. EPP, extended pleurectomy (E/P), and pleurectomy/decortication (P/D) were performed in 31, 20, and 25 cases, respectively. Patients’ demographics and characteristics in the three groups are shown in Table 1.
Full Table
Preoperative computer tomography (CT) of the chest and abdominal ultrasonograph (USG) were taken in all cases. Routine blood examinations and functional evaluation of the respiratory system, with or without diffusing capacity of the lung (DLCO) and ventilation quotient (V/Q), scanning, and cranial magnetic resonance imaging (MRI)/CT were also performed in all cases. The operability was evaluated either clinically or videothoracoscopically, based on performance status, pulmonary function, and staging. Echocardiography or cardiac MRI were performed when found to be necessary. Since 2007, positron emission tomography (PET) has routinely been performed in almost all EPP and E/P cases.
In all cases, the diagnosis was made by pleural biopsy or by video-assisted thoracoscopic surgery (VATS). Pathologic diagnosis was based on standard histologic, histochemical, and immunohistochemical criteria in all cases. Histopathological definitions and assessments were based on the 2004 WHO lung and pleural tumour classification (4). As a positive marker of immunohistochemistry for MPM, standard immunohistochemical markers included calretinin, mesothelin, cytokeratin 5/6, and D2-40. As negative MPM, we used thyroid transcription factor-1, carcinoembryonic antigen and BerEP4. In cases before positive mesothelial markers were available, negative markers were used for making the diagnosis of MPM.
Talc pleurodesis was performed for the cases that were not considered suitable for surgery due to their general condition after diagnosis; These cases were excluded from the study. During this period cases with incomplete pleurectomy for surgical pleurodesis were also excluded from the study. So, cases within the study group were operated for therapeutic purposes.
Mediastinoscopy was performed routinely for EPP and E/P candidates that had pathologic lymph nodes detected by CT or PET. For some of the patients who did not have pathologic lymph nodes detected by CT or PET, however, mediastinoscopy was performed or not performed according to the surgeon’s preference. In cases of lymph node positivity proven by mediastinoscopy, operative plan was changed to P/D in cases candidate to EPP or E/P.
Surgical procedures: in choosing a case, the patient’s Karnofsky index (preferably greater than 80%), cardiopulmonary status, and radiological stage were evaluated. Previously, we gave preference to EPP; in the last five years, E/P rather than EPP was the preferred technique. The personal preference and approach of the surgical team was an important consideration in determining the most appropriate surgical procedure. Also, preference of the patients was taken into consideration in choosing the surgical approach especially for aggressive approach, when told, in recent years.
EPP was performed using the standard technique. Posterolateral thoracotomy was performed through the fifth intercostal space. Mediastinal lymph node sampling was performed to paratracheal, paraesophageal area and internal mammary artery lodge and frozen section examinations were accomplished while decortication in order to modify the operative plan in case of positivity. These mediastinal node positivity was briefly mentioned as N2 disease within the text. For diaphragmatic resection, seventh or eighth intercostal space thoracotomies were performed through the same incision. All the parietal pleura, the lungs, the pericardium, and the ipsilateral diaphragm were resected en bloc following extrapleural dissection. The diaphragm was allocated from the chest wall without leaving residual tissue. Complete dissection was accompanied by reconstruction of hiatus. The reconstruction of the pericardium and ipsilateral diaphragm was done using MERSILENE Polyester Fiber Mesh (ETHICON, LLC).
Mediastinal lymph node dissection was carried out regardless of which surgical procedure was chosen. It was performed following the same procedure we used in non-small-cell lung cancer (NSCLC) cases. In order to prevent locoregional recurrence, prior to the resect VATS incision site (or sites), full-layer excision was included in the surgical procedure for all cases.
E/P was performed consistently with the standard EPP procedure, except for using lung-protecting techniques. Peeling of the visceral pleura was performed when it was infiltrated. The pericardium, and the ipsilateral diaphragm were resected en bloc following extrapleural dissection and reconstructed in usual manner as in EPP.
P/D was carried out by posterolateral thoracotomy in cases at the early stages with minimal or no visceral pleural involvement. Peeling of the visceral pleura was performed when necessary following resection of parietal pleura. P/D was also preferred alone on patients with poor respiratory or hemodynamic status or in patients who refused an aggressive approach. Postoperatively, all patients received adjuvant chemotherapy. The chemotherapy combination consisted of carboplatin and paclitaxel. Following EPP, adjuvant chemoradiation was carried out. Neoadjuvant chemotherapy was not applied to any of the patient within study group. Follow up of all patients was through routine visits and phone calls. The mean follow-up time was 21.9±16.3 months (0-75 months).
Statistical analysis: data are presented as a mean ± standard error of the mean. Continuous numerical data were compared between the two groups using a Student’s t-test or Mann-Whitney U-test. For comparison of non-continuous (categorical) data, the chi-square test or Fisher’s exact test analyses were used. Patients’ survival was expressed by the Kaplan-Meier method (univariate analysis) and the Cox proportional hazards model (multivariate analysis), using the day of transplant as time zero and death time, if death occurred, as the end point. Differences in survival were determined by log-rank test univariate analysis, and prognostic factors having P-value less than 0.1 were included in multivariate analysis. Hazard ratios of the prognostic factors were given with 95% confidence intervals (CI). Hospital mortality was not included to survival analyses. All data analyses were conducted using the Statistical Product and Service Solutions (SPSS 11.0) software. A P-value of less than 0.05 was considered statistically significant.
Results
The median survival time was 20 months in all patients. Overall, five-year survival rate was 14.3% (Figure 1A).
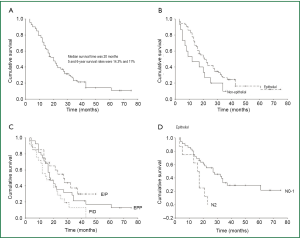
In 60 (78.9%) patients, the malignancy was epithelioid mesothelioma and was of another type in 16 (21.1%) cases (biphasic mesothelioma in 10 patients and sarcomatoid mesothelioma in 6 patients). In five of the biphasic mesothelioma and 3 of sarcomatous mesothelioma the initial diagnosis were epithelioid but pathologic examinations of the surgical specimen revealed that they were biphasic or sarcomatous type. The five-year survival rates for patients with epithelioid mesothelioma were 16% and 0% for patients with other mesotheliomas. The survival rate was significantly better in epithelioid mesothelioma (P=0.049, Figure 1B). The five-year survival rate was 15% in cases when the tumor was located on the right side, and 13% when it was located on the left side. There were no statistically significant differences in relation to tumor location (P=0.652, Table 2).

Full Table
For EPP cases, the median survival rate was 17 months, and the three-to-five year survival rates were 21% and 17%, respectively. For E/P cases, the median survival rate was 27 months and the three-year and four-year survival rates were 34% and 30%, respectively. For P/D cases, the median survival rate was 15 months and the three and five year survival rate was 13% and 0%. There were no statistically significant differences between the three surgical techniques (P=0.088, Figure 1C). A comparative analysis of EPP with E/P (P=0.305), EPP with P/D (P=0.177), and E/P with P/D (P=0.032) indicates only a statistically significant difference in the E/P and P/D comparison (Table 2).
There was no statistically significant difference between the patients’ T-factors (P=0.802, Table 2).
The five-year survival rate was 29% in N0-1 cases. In N2 cases, there were no cases of two-year survival. The survival rate in N2 was comparatively much lower, which was statistically significant (P=0.005, Figure 1D, Table 2). Five of N2 cases among 16 cases were within EPP group. Three of five cases were non-mediastinoscopy group and the remaining two were false negative mediastinoscopic frozen section patients. Eleven of 16 N2 cases were in P/D group.
When the subjects were grouped according to age, gender, cell type, type of the surgery, and N status and evaluated together in multivariate analysis, only P/D (OR 0.3, 95% CI: 0.1-0.9, P=0.049.) and N2 (OR 1.6, 95% CI: 0.9-2.6, P=0.090) were identified as negative prognostic factors (Table 2).
The 30-day general mortality rate was 6.7% (n=5) in the cases studied. The mortality rate was 12.9% (n=4) for patients in the EPP group, 4% (n=1) for those in the P/D group, and 0% for those in the E/P group. Despite hospital mortality was high in EPP group, there was no significant difference between three surgical techniques (P=0.145). The causes of the 30-day hospital mortality in EPP patients were bronchopleural fistula followed by pneumonia and acute respiratory distress syndrome (ARDS) in three cases, and myocardial infarction (MI) in one case. The reasons for mortality in the P/D group were pneumonia, respiratory failure, and multi-organ failure (MOF).
The major complication rate for all cases was 48.07%. The factors were cardiac failure and arrhythmia in nine cases in which positive inotropic support was necessary (33%), prolonged air leakage in six cases, pneumonia in six cases, and bronchopleural fistula in five cases. There was wound infection in four cases, postoperative hemorrage in four cases, pneumonia in two cases, empyema in four cases, and chylothorax in three cases. There were three cases of MI, three cases of sepsis and MOF, two cases of pulmonary embolism, two cases of acute renal failure, and one case each of ARDS, recurrent laryngeal nerve injury, intestinal obstruction, and diaphragmatic patch rupture.
Discussion
This study is a single-center retrospective study that covers patients that have had oncological treatment after surgery for MPM. When deciding on study participants, the first challenge was choosing patients while considering all surgical cases, with anatomical, oncological, and physiological factors that applied to selected cases. Identification of surgery as the option for selected patients was another challenge. There are as many different interdisciplinary insights and applications in the treatment of MPM as they are different surgeons. The surgical techniques of P/D and E/P are being preferred over EPP to a greater extent than in former years, possibly because the MARS study concluded that EPP offers no benefits to MPM patients (1). The preference between P/D and E/P depends as much on surgical tendency, personal choices, and perspective for MPM as on scientific findings. In this paper, we tried to discuss short and long term results of EPP, E/P ve P/D.
EPP, which is radical approach and is one of the surgical branches for the treatment of MPM, was the primary means of surgical management in the 1970s (5). Although the mortality rate is less than 10% in most studies, it has recently emerged as a technique with high surgical morbidity and mortality. It has long been debated whether a complete resection (R0) can be achieved in MPM cases (6,7).
EPP is a serious operation in terms of surgical technique and the trauma suffered by the patient during radical surgery and from the loss of a vital organ. Patients have severe cardiopulmonary/hemodynamic overload. Fluid and electrolyte balance disorders may develop. Among the 31 patients that underwent EPP in our clinic, three patients (9%) developed cardiac dysrhythmias and/or hypotension, and related morbidity was seen in six cases (21%), which led to mortality. Compression after resection of the pericardium by using a pericardial patch placed on the heart or compression or irritation of the myocardium can result in hypertension or hypotension caused by fluid and electrolyte imbalance. In our clinic, postoperative hypertension or arrhythmia was also found in cases that underwent E/P or P/D (Figure 2).
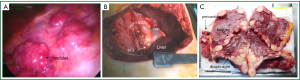
In the presented study, the mortality rate for EPP was 12.9%. When EPP was first devised in the 1970s, the mortality rate was 30% and few surgeons adopted this operating procedure (8) but today the mortality rate for EPP is lower than 5% in some centers.
In most studies, the five-year survival rate under trimodal therapy is reported at less than 20%. The best survival result of our study is the three-year survival rate in the E/P group. E/P is a less aggressive alternative to EPP and can even give better results. It is a more parenchyma-saving surgical technique for tumor eradication when there is lack of parietal pleural invasion, so there in minimal or no parenchymal involvement and visceral pleural invasion. In our study, although the median survival time was similar for both EPP and P/D, the three-year survival time was in favor of EPP. Any hypothesis for the advantages of EPP or E/P can be a better control for local diseases (9,10). We did not apply induction chemotherapy in our study, although it is emphasized as not increasing surgical risk and is well-tolerated (10-13).
EPP was preferred in cases that had lung parenchyma and fissural invasion. E/P was preferred in cases where standard pleurectomy was technically inappropriate, mainly over the diaphragm, when lung parenchyma was intact and applicable for effective cytoreduction of the visceral pleura. In recent years, E/P is preferred for cases in which there was no invasion of lung parenchyma, because of its more efficient local control. E/P offers a hopeful alternative to EPP in patients with poor performance or insufficient cardiopulmonary reserve (14,15) and we had better results with the E/P group of patients. Nevertheless, personal preferences and the approach of the surgical team are more appropriate means to identify the most effective surgical method.
Some of the positive and negative prognostic factors for MPM have long been known (11,16-18). Our study shows that females have a better prognosis; the findings indicate a median survival time of 17 months for males and 31 months for females.
As epithelioid mesothelioma is already known to have better survival chances, EPP and E/P are preferred in patients with epithelioid mesothelioma (16-20). In our study, five patients were to undergo EPP after the preoperative diagnosis of epithelioid mesothelioma, but the pathological examination showed biphasic mesothelioma, indicating a different surgical preference.
Another significant piece of data that we obtained from our study is the poor survival rate of N2 disease (mediastinal lymph node involvement). As shown in Table 2, the median survival time was 27 months for undetermined N2 cases and 16 months for determined N2 cases (P=0.005). Similarly, the three-year survival rate in undetermined N2 cases was 33% and 0% for cases with N2. In the literature, there are authors who perform mediastinoscopy routinely (21) as well as ones who do not perform mediastinoscopy (22) or do it selectively (23). We recommend mediastinoscopy after viewing the results in our patients that underwent EPP and E/P.
Recurrence of MPM is almost inevitable and is well documented. As recurrences are generally local, EPP and E/P may control local disease better (24-26). Intensity-modulated radiation therapy (IMRT), which is applied after EPP, provides an efficient local control, although the role of EPP in MPM and in providing local control is still debated. IMRT seems to provide an additional treatment option, and clinical studies have provided encouraging results in the combined modality approach (10,27). P/D is a less aggressive surgical option and involves lower morbidity and mortality. In most studies, the results after P/D are comparable to or even better than the results after EPP; this may be related to the criteria for choosing patients or successes in adjuvant therapy (28).
Although the aim in EPP is maximal cytoreduction, the loss of lung is inevitable. However, we found intra-abdominal recurrence in nine cases (9 of 49, 18%) during follow-up, which included patients that had undergone EPP and E/P. We believe that the most important factor was implantation metastases that occurred during diaphragmatic resection. In our opinion, the peritoneum that is resected with the diaphragm is a good barrier against implantation of tumor. It is quite difficult to protect the peritoneum during a resection of the diaphragm, especially in the membranous section. It is often perforated, but it can be repaired with sutures. Washing the abdominal cavity with distilled water or applying perioperative intra-abdominal chemotherapy in peritoneum resected cases might be a good therapeutic approach.
The long-awaited MARS study, which was published in 2011, had a significant impact on opinions about EPP (1). EPP was defined as a dangerous and redundant operation on the basis of a randomized study on MPM cases. However, the study had certain limitations, such as the hypothetical deductions made on the basis of a feasibility study to test performing a trial to assess EPP in the management of MPM, the late timing of randomization, the limited number of subjects, and the study methodology (1). Unlike the MARS trial that primarily analyzed the survival effects of chemotherapy, our non-randomized study primarily focuses on the effects of EPP and also of E/P.
Our study finds lower mortality and survival rates in EPP and E/P cases compared to the MARS study. We had a mortality rate of 12% in the 33 cases included in our EPP group. Although this is a relatively high percentage, this value is considerably lower than that reported by the MARS study (12% versus 18%). Within the last years, we were particularly careful in our dissection technique and oncologically elaborate based on our prior experiences. We aimed to separate the diaphragm without leaving residual tissue on the wall and take control of the sinuses through a second thoracotomy at the seventh or eighth intercostal space. We paid particular attention to dissection on both sides, and especially on the inferior vena cava hiatus.
The object of the MARS feasibility trial was to determine whether radical surgery after induction chemotherapy is better than chemotherapy alone. It revealed that survival after chemotherapy alone was better and less associated with adverse events than chemotherapy with radical surgery (1,21). Surgery was found to offer no benefit and to even harm the patients.
The MARS study had its impact on us as surgeons and researchers. As a result, we did not consider EPP suitable for parenchymal involvement, unpaired cases of visceral pleura invasion, or cases of the tumor extending into the fissure. These approaches revived radical P/D for select cases. In Hiddinga’s studies (6), the results of P/D were reported as better than those following EPP, and EPP with preoperative chemotherapy was shown as better than standard EPP. However, in the light of our findings, we have to reconsider the results of the MARS study, because we got better results than the study’s P/D group that had preoperative chemotherapy, which directed us to P/D or E/P. We believe that EPP is a method that should not be ignored, but should only be applied in selected and limited cases.
Our study, which is an evaluation of the surgical methods for MPM, is a retrospective study, which did not include randomization. Notably, the MARS study was not designed to determine whether or not EPP was beneficial for MPM patients, but only to determine the feasibility of such a trial. Weder and Opitz (28) reported that the data in the MARS study does not support its conclusions and it misdirected the clinical outcomes related to MPM studies.
We acknowledge that our study has some limitations. It is numerically limited, with retrospective analysis; additionally, there is the lack of uniform oncologic therapy. Although it was difficult to have data showing rate of local recurrences because of several oncology departments the patients were referred, we strictly have data on their lives by ID numbers. Statistical data may not be significant in a small group of participants. Nevertheless, we hope that it paves the way for further research on the efficacy of radical therapies such as EPP in the treatment of MPM.
In future, there will be an infinite number of MPM studies that focus on crossing surgical techniques, the features that belong to adjuvant treatment, radiotherapy models or the combinations of chemotherapy protocols instead of neoadjuvant therapy. Immunotherapy and gene therapies are further areas of study. The further studies will obviously expand the database and more detailed information on T and N factors will be taken (29). So, better choices on surgical options could be held away from subjectivity. Innovations in classification including wider database studies could be more desicive in choosing the surgical technique. It should not to be forgotten that MPM in which multimodal therapy is the key factor. It is inevitable to take care of the good and poor prognostic factors.
Although MPM is a serious and complex problem that is related to all medical disciplines as well as to thoracic surgery, it is our considered opinion that there positive prognostic factors. Surgery, including radical forms such as EPP, must be considered part of multidisciplinary treatments, in specialized centers (30), though it is difficult to achieve a consensus on this view. However, E/P could be encouraged to EPP with lower mortality rate (0% vs. 12.9%), better survival rate (37% vs. 21% at 3-year). In N2 cases within the presented study, there were no cases of two-year survival. The survival rate in N2 was comparatively much lower, which was statistically significant (P=0.005). So, we thought that N2 cases should not be operated on for EPP or E/P. The decision-making capabilities and expertise of individual surgeons will always be a factor, and any multimodal therapy for MPM will require a prognosis determinant and prospective multi-centered studies that provide alternative therapy models.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. [PubMed]
- Tokat AO, Kutlay H. Gene therapy in malignant mesothelioma. Tuberk Toraks 2003;51:456-60. [PubMed]
- Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999;117:54-63; discussion 63-5. [PubMed]
- Travis WD, Brambilla E, Muller-Hermelink HK, et al. eds. WHO classification: pathology & genetics, tumours of the lung, pleura, thymus and heart. Lyon: IARC Press, 2004.
- Butchart EG, Ashcroft T, Barnsley WC, et al. Pleuropneumonectomy in the management of diffuse malignant mesothelioma of the pleura. Experience with 29 patients. Thorax 1976;31:15-24. [PubMed]
- Hiddinga BI, van Meerbeeck JP. Surgery in mesothelioma--where do we go after MARS? J Thorac Oncol 2013;8:525-9. [PubMed]
- Federico R, Adolfo F, Giuseppe M, et al. Phase II trial of neoadjuvant pemetrexed plus cisplatin followed by surgery and radiation in the treatment of pleural mesothelioma. BMC Cancer 2013;13:22. [PubMed]
- Curran D, Sahmoud T, Therasse P, et al. Prognostic factors in patients with pleural mesothelioma: the European Organization for Research and Treatment of Cancer experience. J Clin Oncol 1998;16:145-52. [PubMed]
- Stewart DJ, Martin-Ucar A, Pilling JE, et al. The effect of extent of local resection on patterns of disease progression in malignant pleural mesothelioma. Ann Thorac Surg 2004;78:245-52. [PubMed]
- Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:3007-13. [PubMed]
- Fahrner R, Ochsenbein A, Schmid RA, et al. Long term survival after trimodal therapy in malignant pleural mesothelioma. Swiss Med Wkly 2012;142:w13686. [PubMed]
- Weder W, Kestenholz P, Taverna C, et al. Neoadjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. J Clin Oncol 2004;22 Suppl 1:3451-7. [PubMed]
- Flores RM. Induction chemotherapy, extrapleural pneumonectomy, and radiotherapy in the treatment of malignant pleural mesothelioma: the Memorial Sloan-Kettering experience. Lung Cancer 2005;49:S71-4. [PubMed]
- Martin-Ucar AE, Nakas A, Edwards JG, et al. Case-control study between extrapleural pneumonectomy and radical pleurectomy/decortication for pathological N2 malignant pleural mesothelioma. Eur J Cardiothorac Surg 2007;31:765-70; discussion 770-1. [PubMed]
- Rusch VW. Pleurectomy/decortication in the setting of multimodality treatment for diffuse malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg 1997;9:367-72. [PubMed]
- Borasio P, Berruti A, Billé A, et al. Malignant pleural mesothelioma: clinicopathologic and survival characteristics in a consecutive series of 394 patients. Eur J Cardiothorac Surg 2008;33:307-13. [PubMed]
- Edwards JG, Abrams KR, Leverment JN, et al. Prognostic factors for malignant mesothelioma in 142 patients: validation of CALGB and EORTC prognostic scoring systems. Thorax 2000;55:731-5. [PubMed]
- Herndon JE, Green MR, Chahinian AP, et al. Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the Cancer and Leukemia Group B. Chest 1998;113:723-31. [PubMed]
- Flores RM, Zakowski M, Venkatraman E, et al. Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J Thorac Oncol 2007;2:957-65. [PubMed]
- Yan TD, Boyer M, Tin MM, et al. Prognostic features of long-term survivors after surgical management of malignant pleural mesothelioma. Ann Thorac Surg 2009;87:1552-6. [PubMed]
- Treasure T, Waller D, Tan C, et al. The Mesothelioma and Radical surgery randomized controlled trial: the Mars feasibility study. J Thorac Oncol 2009;4:1254-8. [PubMed]
- Lindenmann J, Matzi V, Neuboeck N, et al. Multimodal therapy of malignant pleural mesothelioma: is the replacement of radical surgery imminent? Interact Cardiovasc Thorac Surg 2013;16:237-43. [PubMed]
- Trousse DS, Avaro JP, D’Journo XB, et al. Is malignant pleural mesothelioma a surgical disease? A review of 83 consecutive extra-pleural pneumonectomies. Eur J Cardiothorac Surg 2009;36:759-63. [PubMed]
- Flores RM. Surgical options in malignant pleural mesothelioma: extrapleural pneumonectomy or pleurectomy/decortication. Semin Thorac Cardiovasc Surg 2009;21:149-53. [PubMed]
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6, 626.e1-3.
- Weder W, Stahel RA, Bernhard J, et al. Multicenter trial of neo-adjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. Ann Oncol 2007;18:1196-202. [PubMed]
- Lang-Lazdunski L, Bille A, Lal R, et al. Pleurectomy/decortication is superior to extrapleural pneumonectomy in the multimodality management of patients with malignant pleural mesothelioma. J Thorac Oncol 2012;7:737-43. [PubMed]
- Zucali PA, De Vincenzo F, Simonelli M, et al. Future developments in the management of malignant pleural mesothelioma. Expert Rev Anticancer Ther 2009;9:453-67. [PubMed]
- Rusch VW, Giroux D. Do we need a revised staging system for malignant pleural mesothelioma? Analysis of the IASLC database. Ann Cardiothorac Surg 2012;1:438-48.
- Cao C, Tian D, Manganas C, et al. Systematic review of trimodality therapy for patients with malignant pleural mesothelioma. Ann Cardiothorac Surg 2012;1:428-37.


