Refractory hypertension with massive proteinuria may be reversed in renal artery stenosis patients with low proteinuria selectivity index after stenting
Introduction
It is well known that renal artery stenosis causes refractory hypertension and renal dysfunction. Renal artery revascularization by stening with percutaneous renal artery angioplasty (PTRA) or surgery is used to cure hypertension or to improve renal function. However, recent randomized studies have indicated less favorable results, with only little difference in blood pressure control or renal function in selected groups of patients treated with endovascular correction of stenosis or only pharmacologically (1). It is reported that early identification and management of atherosclerotic renovascular stenosis (ARAS), especially PTRA followed by primary stenting, have a beneficial effect on the control of hypertension and protecting the renal function (2). However, up to now, there was no sufficient biochemical or instrumental parameters to predict which patients would benefit from revascularization or who would respond with major adverse events. Hence, the main problem is still to find criteria to correctly select patients. A highly selective proteinuria (selectivity Index, SI <0.1) has been suggested to be associated with a less severe lesion and predict a negative clinical outcome (3,4). Using the criteria of SI, we hereby describe our choice of examinations and treatments in a male patient with renovascular hypertension associated with massive proteinuria, and discussed the possible relation between SI and the clinical outcome after percutaneous revascularization in this case.
Case report
A 68-year-old man was referred to our hospital for examination and treatment of hypertension associated with progressive renal dysfunction in Dec. 2009. He had been diagnosed with hypertension for more than twenty years, and his BP was suboptimally under controlled after using multiple antihypertensive drugs. In 2008, he was admitted to our hospital for chronic low back pain, and abdominal aortic aneurysm was then diagnosed by CTA scan. In Jan. 2009, a stent was placed into his abdominal artery. In Sep. 2009, his BP level had suddenly elevated to 190/110 mmHg, sometimes in association with headache. Addition of amlodipine (5 mg, qd) and Valsartan (80 mg, qd) was not effective. And he admitted to our hospital again for further examination and treatment of hypertension.
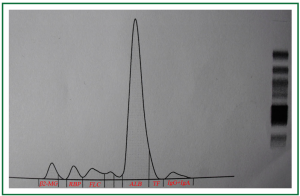
Physical examination on admission showed high blood pressure (178/99 mmHg), his serum BUN, Cr and uric acid was 10.33 mmol/L, 227.4 μmol/L and 505.9 μmol/L, respectively. The Cr clearance had decreased to 34.5 mL/min, and the urinalysis revealed that his urine protein has increased to 1.404 g/24 hours. The renal Doppler ultrasonography examination further revealed a heavy stenosis at the ostia of the right renal arteries, and the renal resistance index (RI) was 0.84 (Figure 1). Then the valsartan was stopped using, Carvedilol (5 mg/day) and double dosage of amlodipine (10 mg/day) was introduced to control his blood pressure. However, his renal function deteriorated progressively, in Jan. 2010, his serum Cr level had increased to 343.8 μmol/L, and the Cr clearance was only 35.1 mL/min. Notably, a massive proteinuria (4.343 g/24 h) was observed at that time. In order to know the composition of his urine protein, his urine sample was electrophorized, and 75% albumin, 5.7% free light chain, and 3.1% IgG, IgA was detected (Figure 2). Then the concentration of his urinary and serum IgG and albumin was measured, and the caculated proteinuria SI based upon IgG [SI, the formula: SI = uIgG × sAlb/sIgG × uAlb (3,5)] was 0.08. Furthermore, those indicators for diagnosing the autoimmune disease like serum ANCA, ENA and dsDNA was examined and fortunately, all of those indices were negative.

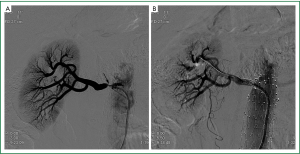
Therefore, PTRA was performed and renal angiography documented 85-90% narrowing at ostia of right renal artery (RRA) (Figure 3A), with no narrowing at the left renal artery (LRA). Using a guiding catheter and a guidewire, the stenosis at RRA was passed, and a balloon-expandable renal stent (6.0/15 mm) was implanted without residual stenosis (Figure 3B). A few days after renal artery stenting, his blood pressure gradually improved and antihypertensive medications were decreased. His serum Cr also decreased after the PTRA procedure. Surprisingly, after 2-year follow up, his renal function kept steady in a relative normal conditions, with serum Cr decreased to 127.3 μmol/L, and the Cr clearance increased to 71.9 mL/min. His uric acid was 477.5 μmol/L, and his blood pressure was controlled in 135/85 mmHg with the adminstration of 20 mg olmesartan and 5 mg amlodipine per day. Furthermore, the renal Doppler ultrasonography examination demonstrated that his RI decreased to 0.33 (Figure 1B). In addition, his urine protein was only 0.68 g/24 h at that time, and the follow-up study is still going on.
Disscussion
Renal artery stenosis is usually caused by atherosclerosis, it is a common problem in patients with atherosclerosis in other vascular distributions and is well recognized as a cause of sencondary hypertension and renal insufficiency. Renal artery revascularization by stening with PTRA or surgery is used to control hypertension or to improve renal function in those patients. And current practice guidelines for revascularization in those patients with significant RAS list a class IIa indication for resistant (uncontrolled despite 3 antihypertensive medications), malignant, or accelerating hypertension, and chronic renal insufficiency with bilateral RAS (6). However, the results from a recent meta-analysis may be a little frustrating, which evaluated six randomized controlled trials of whether percutaneous revascularization the RAS patients had the additional clinical benefits other than the improvement in blood pressure control. The authors found that in RAS patients, PTRA with or without stent may only result in a lower requirment for antihpertensive drugs, but had no benefits for decreasing the serum creatinine or improving clincal outcomes (1). It seems counterintuitive that re-open the stenosis with stent surely can improve the renal blood flow, but this improvement was not associated with the better clinical outcomes. This contradiction might explained by the following reasons: firstly, those conditions resulting in atherosclerotic narrowing of the renal arteries can also lead to parenchymal renal injury, and in such conditions, the renal function was more often influenced by underlying microvascular kidney disease. Therefore, in late disease stage, even if the blood flow was improved, the renal function and clinical outcome may not become better. Secondly, RAS can activate the renin-angiotensin system, which can results in excess inflammatory mediators and reactive oxygen species, and all of those mediators can result in irreversible glomerular damage (1). As a result, if those patients with a less renal injury could indentified, they might benefit from the revascularzation therapy not only in blood pressure controlling but also in correcting the renal dysfunction and clinical outcomes.
In this case, rapid declined renal function and massive proteinuria was observed, and someone argued that a long history of high baseline creatinine (serum creatinine >300 μmol/L), high RI level (>0.8), and severe proteinuria (>1 g/day) prognosis adverse clinical outcomes for revascularization (7,8). However, proteinuria is commonly seen in patients with RAS, and the pathophysiology of this phenomenon is thought to be hormonal and hemodynamic disturbances (9). In fact, the qualitative aspects of proteinuria may give us more information than the simple measurement of 24-hour proteinuria, which is a reliable indicator for identifying in the single patient the degree of the damage that takes palace in the glomerular and tubulointerstitial compartments of nephron (4,10). In our study, we then chose the proteinuria SI based upon IgG to evaluate the severity of the renal injury in this patient. The presence of highly selective proteinuria (SI <0.1) reflects a less severe lesion, the moderately selective (SI: 0.11-0.20) and nonselective (SI >0.2) proteinuria, although not different from highly selective proteinuria in terms of total 24-hour protein loss, are associated with severe lesion and nonreversible clinical outcome (3,5,10). The SI of our patient was only 0.08, which indicated that his proteinuria was a highly selective proteinuria, and we supposed that revascularization of the renal artery might help him to gain a dialysis-free life and a well-controlled blood pressure. In two-years of follow-up, the revascularization procedure did be beneficial to these patients not only in controlling the blood pressure but also in improving the renal function.
Based on this observation coupled with previous reports, we tentatively suggest that SI might be a simple prognostic factor for ARAS patient who undergo the revascularization therapy. Of course, as only one patient this case is, this conclusion is still awaiting more clinical observations to confirm.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (NSFC 30900602) and the National Natural Science Foundation of Jiangsu Province (SBK2012879) to Dr. Wang Junhong and (SBK2011382) to Pro. Guo Yan.
Disclosure: The authors declare no conflict of interest.
References
- Kumbhani DJ, Bavry AA, Harvey JE, et al. Clinical outcomes after percutaneous revascularization versus medical management in patients with significant renal artery stenosis: a meta-analysis of randomized controlled trials. Am Heart J 2011;161:622-30.e1.
- Dorros G, Jaff M, Mathiak L, et al. Multicenter Palmaz stent renal artery stenosis revascularization registry report: four-year follow-up of 1,058 successful patients. Catheter Cardiovasc Interv 2002;55:182-8. [PubMed]
- McQuarrie EP, Shakerdi L, Jardine AG, et al. Fractional excretions of albumin and IgG are the best predictors of progression in primary glomerulonephritis. Nephrol Dial Transplant 2011;26:1563-9. [PubMed]
- Bakoush O, Torffvit O, Rippe B, et al. High proteinuria selectivity index based upon IgM is a strong predictor of poor renal survival in glomerular diseases. Nephrol Dial Transplant 2001;16:1357-63. [PubMed]
- Liu FY, Li Y, Peng YM, et al. Relationship between clinical predictors and tubulointerstitial damage in adult-onset primary nephrotic syndrome. Arch Med Res 2006;37:981-6. [PubMed]
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol 2006;47:1239-312. [PubMed]
- Eklöf H, Bergqvist D, Hägg A, et al. Outcome after endovascular revascularization of atherosclerotic renal artery stenosis. Acta Radiol 2009;50:256-64. [PubMed]
- Campo A, Boero R, Stratta P, et al. Selective stenting and the course of atherosclerotic renovascular nephropathy. J Nephrol 2002;15:525-9. [PubMed]
- Wadhwa A, Kazory A. Nephrotic range proteinuria associated with unilateral renal artery stenosis. Int Urol Nephrol 2008;40:821-2. [PubMed]
- Bazzi C, Petrini C, Rizza V, et al. A modern approach to selectivity of proteinuria and tubulointerstitial damage in nephrotic syndrome. Kidney Int 2000;58:1732-41. [PubMed]


