Pattern of lymph node metastases of squamous cell esophageal cancer based on the anatomical lymphatic drainage system: efficacy of lymph node dissection according to tumor location
Introduction
Despite recent advances in multidisciplinary approaches including radio- and chemotherapy, surgical resection remains the standard treatment for potentially resectable esophageal carcinoma (1,2). Postoperative outcomes for patients with esophageal carcinoma are determined by the extent of the primary tumor and the lymphatic spread of the disease. Lymph node status is a particularly strong predictor of survival and recurrent disease (3).
Some patients can be cured by removal of the regional disease with lymph node metastases, and removal of all potentially involved regional lymph nodes is essential to achieve cure in these patients (4). Considerable controversy remains regarding the extent of lymph node dissection in esophageal cancer surgery. Some favor more limited resection strategies such as transhiatal resection or transthoracic resection with minimal nodal dissection and hold that extended nodal dissection offers no meaningful improvement in survival (5,6). Others have argued that three-field lymphadenectomy entailing nodal dissection along the recurrent laryngeal nerves in the thoracic cavity extending to the “third” field in the neck improves local disease control and possibly enhances survival (7-10). Adequate preoperative and/or intraoperative assessment of the patient's lymph node status can help tailor the extent of the resection. However, even with latest diagnostic techniques, metastases in lymph nodes may escape detection, as it is extremely difficult to detect all the positive nodes (11-13). Therefore, a thorough understanding of pattern of lymph node metastases is important.
Knowing the anatomical lymphatic drainage system of the esophagus is crucial to understanding the dissemination pattern of esophageal tumor cells. The basic concept of radical lymph node dissection for gastrointestinal cancers is radical dissection of lymphatic spread in the mesentery. In this review, the anatomical bases of lymphatic drainage system in the mesentery of the esophagus (i.e., mesoesophagus) is considered and verified using clinical data of lymph node metastasis.
Embryology of the esophagus and the mesentery of the esophagus
Lymphatic channels of gastrointestinal tracts develop along the mesenteric arteries and nerves in the mesentery. The structure of the mesentery of the esophagus has seldom been discussed, as this tissue is not easily identified (14). However, the embryology of the mesentery can provide information that is useful for identification of the mesentery of the esophagus.
Initially, the foregut, midgut, and hindgut are in broad contact with the mesenchyme of the posterior abdominal wall. The connecting tissue bridge has become narrow, and the caudal part of the foregut, the midgut, and a major part of the hindgut are suspended by the dorsal mesentery. The midgut tube rapidly elongates to increase digestive function. The dorsal mesentery enables the midgut to achieve sufficient length by overcoming the limitations of the dorsal wall. The dorsal mesentery of the jejunal and ileal loops is known as the mesentery proper, and, in the region of the colon, it is known as the mesocolon.
The esophagus is initially very short. At this early stage, the proximal part of the esophagus is held in place by the pharynx, the inferior thyroid vessels, and the vagal nerves. Those vessels and nerves are components of the proximal mesoesophagus. The distal part of the esophagus is held in place by the cardia and by the celiac vessels and nerves. Those vessels and nerves are components of the distal mesoesophagus. During the embryonal growth, the esophagus lengthens passively as the heart and lungs expand. It is unnecessary to have the mesentery and the mesenteric arteries different from the midgut. The middle and lower part of the esophagus stretches as the vascular and lymphatic networks develop in the submucosal layer. The vessels, nerves, and regional lymph nodes of the middle and lower esophagus are located immediately adjacent to the esophagus without growth of the fold like the dorsal mesentery.
Anatomy of the arteries and nerves in of the mesentery of the esophagus
Blood vessels, nerves and lymphatics are distributed to and from the gut wall throughout the mesenteries. Lymphatic channels develop along the arteries and nerves. The cervical and the upper thoracic esophagus are supplied by the inferior thyroid artery. In the distal end of the esophagus, the ascending branches from the left gastric artery and the left phrenic artery supply. The middle and lower thoracic esophagus is supplied mainly by the dense submucosal interconnected network between the proximal and the distal esophagus, and a little by the bronchial and esophageal branches of the thoracic aorta. This allows it possible to perform transhiatal esophagectomy with blunt dissection.
The parasympathetic and sympathetic nerves travel in the vagal nerve to the esophageal wall. Nerve fibers travel via the recurrent laryngeal nerves and supply the upper one-third of the esophagus. The postganglionic sympathetic fibers travel along the inferior thyroid arteries. The lower two-thirds of the esophagus is supplied by the vagal nerve, which passes through the esophageal plexus that surrounds the esophagus at the level of the carina. Nerves from the lower ganglia pass either directly to the esophageal plexus or to the celiac ganglion to innervate the distal esophagus.
Lymphatic drainage of the esophagus
Lymphatic infiltration of cancer generally spreads through lymphatic channels develop along the arteries and nerves in the mesentery toward the central nodes. Therefore, a recognition of the lymphatic drainage system of the esophagus based on the anatomical mesentery is crucial to achieving an adequate field of dissection.
The abundant lymphatic channels in the lamina propria mucosae and submucosa of the esophagus are well known from classic descriptions (15-17). The esophagus stretches passively as the heart and lungs expand as the vascular and lymphatic networks develop in the submucosal layer. An anatomical study of serial transverse thin sections by Kuge et al. showed that long longitudinal lymphatic extension in the esophageal submucosa is very evident (18). The submucosal lymphatics mainly drain in a longitudinal fashion directly to their proximal and distal ends.
Another anatomical study by Mizutani et al. showed a morphological connection between submucosal lymphatic vessels in the proximal esophagus and recurrent nerve nodes in the superior mediastinum (19). This lymphatic route follows branches from the inferior thyroid arteries and the recurrent laryngeal nerves. Those arteries and nerves are components of the mesentery of the proximal esophagus. The lower esophageal segment mostly drains its lymph into paracardial nodes related to celiac axis nodes. This lymphatic route follows ascending branches from the left gastric artery and the left phrenic artery and the anterior and the posterior vagal trunks in the cardia and to the celiac trunks and ganglion. Those arteries and nerves are components of the mesentery of the distal end of the esophagus (Figure 1).
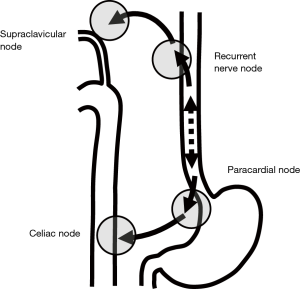
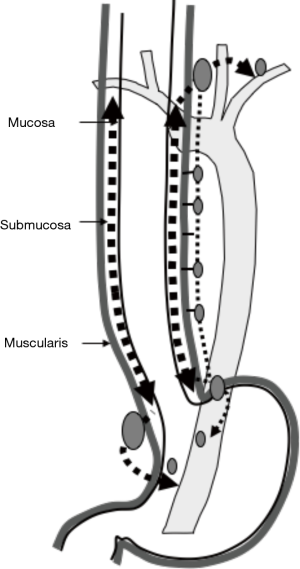
Kuge et al. also reported that lymphatic routes to paraesophageal lymph nodes usually originate from the intermuscular area of the muscularis propria and lymphatic communication between the submucosa and intermuscular area was rarely apparent histologically (18). Lymphatic routes to the mid and lower paraesophageal lymph nodes usually originate from the intermuscular area of the muscularis propria in the middle and lower esophagus. In the mid-portion of the esophagus, lymphatics drain to the proximal esophagus, and then to the superior mediastinum rather than penetrate the muscle layer to reach mid paraesophageal nodes. The lymphatics of the lower thoracic esophagus do not drain to paraesophageal nodes at the same level, but rather drain downwards to the distal end of the esophagus and then to paracardial nodes related to celiac axis nodes along the left gastric artery and the left inferior phrenic artery (Figure 2). These morphologies suggest an explanation for anatomically distant lymph nodes metastasis known as “skip metastasis” in thoracic esophageal cancer.
Clinical feature of lymph node metastasis
Several clinical studies verified this anatomical knowledge for lymph drainage of the esophagus. The anatomical concept was confirmed by a large series of single institution (20) and the nationwide registry in Japan (21). For upper esophageal tumors, upper mediastinal nodes had the highest incidence of metastasis and are the most important dissection target. Supraclavicular nodes had also high incidence. In patients with tumor in the mid esophagus, upper mediastinal nodes had the highest incidence of metastasis followed by paracardial and supraclavicular nodes. Patients with tumor in the lower esophagus had the highest incidence of metastasis in paracardial nodes. However, the incidence of metastasis of upper mediastinal nodes was as high as that of lower mediastinal nodes. The clinical data for the incidence of involved nodes verified the anatomical observations that long longitudinal extension of lymphatic drainage in the submucosa connected to the superior mediastinum along the recurrent nerve and paracardial lymphatics. Isolated distant lymph node involvement is therefore not necessarily a sign of advanced disease (20-22).
Another anatomical concept was also confirmed, with lymphatic routes to mid and lower paraesophageal lymph nodes usually originating from the intermuscular area of the muscularis propria and restricted lymphatic communication between submucosal and intermuscular areas. When tumor was limited to within the submucosal layer, incidences of paraesophageal node metastasis in the mid- and lower mediastinum were very low. Patients with tumor located in the upper esophagus showed node metastasis in the upper mediastinum most frequently as a matter of course (19). Even with tumors located in the mid- and lower esophagus, node metastasis was more frequent in the upper mediastinum and paracardial area than in the mid- and lower mediastinum. In patients with pT2-4 tumor, incidence of lymph node metastasis in the mid- and lower mediastinum was increased dramatically compared with patients showing pT1 tumor. However, incidences of node metastasis in the mid- and lower mediastinum were still lower than those in the upper mediastinum and paracardial area. Upper mediastinal node metastasis was frequent as same as in the lower mediastinum in patients with tumor located in the lower esophagus. When tumors are limited to the submucosal layer in the middle and lower esophagus, tumor cells have little chance to flow into lymphatic routes originating from the intermuscular area of the muscularis propria and to spread to paraesophageal nodes. When tumor invades or penetrates the muscle layer, the incidence of paraesophageal lymph node metastasis in the middle and lower mediastinum increases. Paraesophageal lymph node metastasis in the middle and lower mediastinum is a sign of more advanced esophageal cancer invading the muscle layer (20,21).
Efficacy of LN dissection according to tumor location
Those clinical observations showed that the incidence of metastasis to lymph nodes did not reflect the anatomical distance from the primary tumor, but rather the lymphatic drainage system presented above (18,19). Even with tumors located in the middle and lower esophagus, lymphatic metastasis was frequent in the upper mediastinal and paracardial areas. The conventional hypothesis is that tumor cells involve the nearby nodes first, then spread to nodes a little further, and finally reach distant nodes. The extent of node dissection has been estimated by anatomical distance from the primary tumor to the dissected node area. However, in patients with middle and lower esophageal tumors, the incidence of metastasis in the middle and lower mediastinal area were less than those of upper mediastinal area and paracardial area. Therefor extent of dissection should be not tailored according to the anatomical distance from the tumor but according to the incidence of metastasis.
The incidence of metastasis of each node area was differed by tumor location (21). The areas for node dissection should be modified according to the location of the tumor. For upper esophageal tumors, the upper mediastinal area had the highest incidence of metastasis and is the most important dissection target. In patients with tumor in the middle esophagus, upper mediastinal area had the highest incidence of metastasis followed by paracardial and supraclavicular areas. For patients with tumor in the middle esophagus, the most common type of esophageal tumor in Asia, not only mediastinal and abdominal but also cervical dissection by the three-field approach is recommended. Patients with tumor in the lower esophagus had the highest incidence of metastasis in paracardial area. However, the incidence of metastasis in upper mediastinal area was as high as that of lower mediastinal area. Upper mediastinal dissection is recommended for all patients with thoracic esophageal squamous cell carcinoma, irrespective of the location.
Many patients with lower esophageal tumors and the proximal margin of the tumor in the middle esophagus had metastasis to upper mediastinal and supraclavicular nodes (21). It suggests that the proximal nodal spread to upper mediastinal and supraclavicular nodes is reflected to the location of proximal margin of the tumor. The attention to the proximal margin of tumor should be paid in planning the extent of node dissection. The proximal margin of squamous cell carcinoma tends to be more proximal than those of adenocarcinoma. The upper mediastinal and supraclavicular node metastasis are not neglected.
The nationwide registry showed that the efficacies of node dissection differed by area of lymph node (21). Many previous studies demonstrated that the number of lymph nodes removed is an independent predictor of survival after esophagectomy for cancer (4,23-27). The extent of lymph node dissection in esophageal cancer surgery was estimated by the number of resected regional lymph nodes. In the UICC TNM Classification, it is recommended that histological examination of a regional lymphadenectomy specimen ordinarily include 7 or more lymph nodes (28). The AJCC staging manual recommends that, for pT1, approximately 10 nodes must be resected to maximize survival; for pT2, 20 nodes; and for pT3 or pT4, 30 nodes or more (29) based on the data of the worldwide esophageal cancer collaboration (27). In NCCN guideline, in patients undergoing esophagectomy without induction chemoradiation, at least 15 lymph nodes should be removed to achieve adequate nodal staging (30). However, when only the node areas with low incidence of metastasis are dissected, and those with high incidence of metastasis are not dissected, the efficacy of node dissection is low, even more than 20 nodes are dissected. Thus the effective extent of node dissection should be modified by the incidence of metastasis of node areas.
Conventional dissection is based on the hypothesis that thoracic esophageal tumor cells first involve the nearby paraesophageal nodes. Middle and lower esophageal tumors first involve middle and lower paraesophageal nodes. Then, tumor cells spread to the upper mediastinum and paracardial area. Finally, tumor cells reach distant nodes in the supraclavicular and celiac area. The efficacy of extended dissection appears limited in this advanced situation. Supraclavicular nodes included in distant metastases (M1) (28,29). Most clinicians believe that the presence of M1 disease precludes curative treatment approaches. The presence of supraclavicular node metastasis, similar to visceral organ metastasis, may be considered a contraindication for curative surgery, and supraclavicular node dissection may be considered not associated with any survival benefit. The hypothesis described above was not substantiated by the anatomical studies and clinical data. From the time the tumor is limited to within the submucosal layer, tumor cells can still spread craniocaudally via the submucosal lymphatic plexus and involve nodes in the upper mediastinum along the recurrent nerve and paracardial area. The lymphatic channels connect recurrent nerve nodes to supraclavicular nodes and connect left gastric artery node to celiac node. Patients who had supraclavicular node metastasis appeared to have a better survival than patients with visceral metastasis in several studies (10,31-33). A large series of 1,309 patients who underwent esophagectomy with supraclavicular node dissection for thoracic esophageal cancer was reported (34). The incidence of supraclavicular node metastases was 14.5%. Although patients with supraclavicular node metastases had a worse prognosis than patients without supraclavicular node disease, 24.1% of patients with supraclavicular node disease resected for curative intent (R0) achieved long-term survival. Supraclavicular node status did not predict survival outcome when patients had the same number of metastatic nodes. Survival was worse overall in patients with positive supraclavicular node, but then clearly shows that it is not simply because of supraclavicular node but mostly because of the number of involved nodes. This indicates the survival benefit of dissection of metastases to supraclavicular nodes in patients with thoracic esophageal carcinoma who undergo surgical treatment with curative intent. Therefore, potentially curative resections can still be performed. Like the celiac node, a 1-cm supraclavicular node can be easily removed. However, curative resection is not possible for a massive supraclavicular node. For patients with tumor in the mid esophagus, the most common location of esophageal tumor in Asia, not only mediastinal and abdominal but also cervical dissection by the three-field approach is recommended. Upper mediastinal dissection is recommended for all patients with thoracic esophageal squamous cell carcinoma, irrespective of the location.
The Japanese studies were based on patients with squamous cell carcinoma, and patients with adenocarcinoma were not included. However, in Asian patients, including Japanese patients, squamous cell carcinoma remains the predominant histological cell type of esophageal cancer, and more than half of tumors locate in the upper and middle esophagus.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Swisher SG, Moughan J, Komaki RU, et al. Final Results of NRG Oncology RTOG 0246: An Organ-Preserving Selective Resection Strategy in Esophageal Cancer Patients Treated with Definitive Chemoradiation. J Thorac Oncol 2017;12:368-74. [Crossref] [PubMed]
- Markar S, Gronnier C, Duhamel A, et al. Salvage Surgery After Chemoradiotherapy in the Management of Esophageal Cancer: Is It a Viable Therapeutic Option? J Clin Oncol 2015;33:3866-73. [Crossref] [PubMed]
- Peyre CG, Hagen JA, DeMeester SR, et al. Predicting systemic disease in patients with esophageal cancer after esophagectomy: a multinational study on the significance of the number of involved lymph nodes. Ann Surg 2008;248:979-85. [Crossref] [PubMed]
- Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg 2008;248:549-56. [PubMed]
- Law S, Wong J. Lymph node dissection in surgical treatment of esophageal neoplasms. Surg Oncol Clin N Am 2007;16:115-31. [Crossref] [PubMed]
- Orringer MB, Marshall B, Chang AC, et al. Two thousand Transhiatal esophagectomies: changing trends, lessons learned. Ann Surg 2007;246:363-72. [Crossref] [PubMed]
- Kato H, Watanabe H, Tachimori Y, et al. Evaluation of neck lymph node dissection for thoracic esophageal carcinoma. Ann Thorac Surg 1991;51:931-5. [Crossref] [PubMed]
- Hagen JA, DeMeester SR, Peters JH, et al. Curative resection for esophageal adenocarcinoma: analysis of 100 en bloc esophagectomies. Ann Surg 2001;234:520-30. [Crossref] [PubMed]
- Altorki N, Kent M, Ferrara C, et al. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg 2002;236:177-83. [Crossref] [PubMed]
- Lerut T, Nafteux P, Moons J, et al. Three-field lymphadenectomy for carcinoma of the esophagus and gastroesophageal junction in 174 R0 resections: impact on staging, disease-free survival, and outcome: a plea for adaptation of TNM classification in upper-half esophageal carcinoma. Ann Surg 2004;240:962-72; discussion 972-74. [Crossref] [PubMed]
- Kutup A, Link BC, Schurr PG, et al. Quality control of endoscopic ultrasound in preoperative staging of esophageal cancer. Endoscopy 2007;39:715-9. [Crossref] [PubMed]
- Lowe VJ, Booya F, Fletcher JG., et al. Comparison of positron emission tomography, computed tomography, and endoscopic ultrasound in the initial staging of patients with esophageal cancer. Mol Imaging Biol 2005;7:422-30. [Crossref] [PubMed]
- van Vliet EP, Heijenbrok-Kal MH, Hunink MG, et al. Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer 2008;98:547-57. [Crossref] [PubMed]
- Matsubara T, Ueda M, Nagao N, et al. Cervicothoracic approach for total mesoesophageal dissection in cancer of the thoracic esophagus. J Am Coll Surg 1998;187:238-45. [Crossref] [PubMed]
- Sakata K. Uber die Lymphgefasse des Oesophagus und uber seine regionalen. Lymphdrusen mit Berucksichtigung der Verbreitung des Karcinoms. Mitt Grenzbeg Med Chizg 1903;11:634-56.
- Rouvie’re H. Lymphatics of the larynx, the trachea, and the oesophagus. In: Anatomy of the human lymphatic system. Ann Arbor: Edwards Brothers, 1938:57-62.
- Weinberg JA. Lymphatics of the esophagus. In: Haagensen CD, Feind CR, Herter FP, et al. editors. The lymphatics in cancer. Philadelphia: Saunders, 1972:245-9.
- Kuge K, Murakami G, Mizobuchi S, et al. Submucosal territory of the direct lymphatic drainage system to the thoracic duct in the human esophagus. J Thorac Cardiovasc Surg 2003;125:1343-9. [Crossref] [PubMed]
- Mizutani M, Murakami G, Nawata S, et al. Anatomy of right recurrent nerve node: why does early metastasis of esophageal cancer occur in it? Surg Radiol Anat 2006;28:333-8. [Crossref] [PubMed]
- Tachimori Y, Nagai Y, Kanamori N, et al. Pattern of lymph node metastases of esophageal squamous cell carcinoma based on the anatomical lymphatic drainage system. Dis Esophagus 2011;24:33-8. [Crossref] [PubMed]
- Tachimori Y, Ozawa S, Numasaki H, et al. Efficacy of lymph node dissection by node zones according to tumor location for esophageal squamous cell carcinoma. Esophagus 2016;13:1-7. [Crossref] [PubMed]
- Igaki H, Kato H, Tachimori Y, et al. Cervical lymph node metastasis in patients with submucosal carcinoma of the thoracic esophagus. J Surg Oncol 2000;75:37-41. [Crossref] [PubMed]
- Kang CH, Kim YT, Jeon SH, et al. Lymphadenectomy extent is closely related to long-term survival in esophageal cancer. Eur J Cardiothorac Surg 2007;31:154-60. [Crossref] [PubMed]
- Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable esophageal cancer. J Gastrointest Surg 2007;11:1384-93; discussion 1393-4. [Crossref] [PubMed]
- Altorki NK, Zhou XK, Stiles B, et al. Total number of resected lymph nodes predicts survival in esophageal cancer. Ann Surg 2008;248:221-6. [Crossref] [PubMed]
- Greenstein AJ, Litle VR, Swanson SJ, et al. Effect of the number of lymph nodes sampled on postoperative survival of lymph node-negative esophageal cancer. Cancer 2008;112:1239-46. [Crossref] [PubMed]
- Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg 2010;251:46-50. [Crossref] [PubMed]
- Brierly JD, Gospodarowicz MK, Wittekind C. Union for International Cancer Control (UICC). TNM classification of malignant tumors, 8th ed. West Sussex: John Wiley & Sons, 2017.
- Amin MB, Edge SB, Greene FL, et al. American Joint Committee on Cancer. AJCC Cancer Staging Manual. 8th ed. Switzerland: Springer, 2017.
- Ajani JA, D'Amico TA, Almhanna K, et al. NCCN Clinical Practice Guidelines in Oncology. Esophageal and esophagogastric junction cancers, version 2.2016. J Natl Compr Canc Netw 2016. [Crossref]
- Akiyama H, Tsurumaru M, Udagawa H, et al. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg 1994;220:364-72; discussion 372-3. [Crossref] [PubMed]
- Tachimori Y, Kato H, Watanabe H. Surgery for thoracic esophageal carcinoma with clinically positive cervical nodes. J Thorac Cardiovasc Surg 1998;116:954-9. [Crossref] [PubMed]
- Lee PC, Port JL, Paul S, et al. Predictors of long-term survival after resection of esophageal carcinoma with nonregional nodal metastases. Ann Thorac Surg 2009;88:186-92; discussion 192-3. [Crossref] [PubMed]
- Tachimori Y, Ozawa S, Numasaki H, et al. Supraclavicular node metastasis from thoracic esophageal carcinoma: A surgical series from a Japanese multi-institutional nationwide registry of esophageal cancer. J Thorac Cardiovasc Surg 2014;148:1224-9. [Crossref] [PubMed]


