The development of targeted therapy in small cell lung cancer
Introduction
Lung cancer remains the leading cause of cancer deaths in both males and females in the United States (1). Small cell lung cancer (SCLC) accounts for about 15% of all cases of lung cancer worldwide, is an aggressive neuroendocrine subtype of lung cancer for which there is no effective treatment (2,3). Patients with limited disease (LD) have a median survival time of 16-24 months when treated with chemotherapy and concurrent thoracic radiation (4,5). Chemotherapy remains the standard therapeutic modality for extensive disease (ED), with a median survival of 7-12 months (4). At the same time, prophylactic cranial irradiation (PCI) in chemotherapy-responding patients, allowing a modest gain in disease-free survival (DFS) and overall survival (OS) and decrease the risk of developing brain metastases (6,7). However, no major therapeutic progress has been achieved in SCLC in the past decade and there remains an unmet need for more effective treatments. In recent years, there has been an increase in effort to understand the molecular biology of SCLC and to exploit this knowledge for therapeutic control through the development of so-called targeted therapies (please see Figure 1 and Table 1). This is most attractive because response rates to chemotherapy in SCLC are high, and so less toxic, orally administered treatments to maintain a complete or partial response and prevent or delay relapse would theoretically be of major potential benefit. Here, we summarize potentially viable targets and new agents that have been developed and employed in recent, ongoing and future clinical trials to attempt to improve clinical outcomes in this disease.
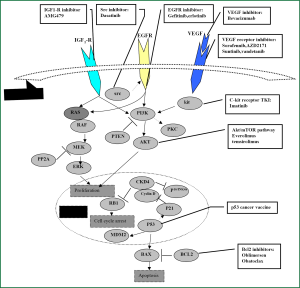
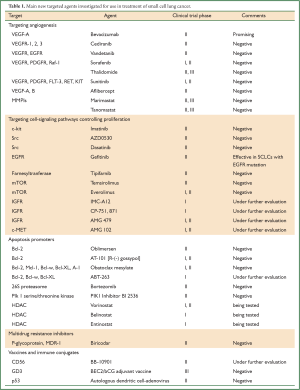
Full Table
Targeting angiogenesis
Angiogenesis is essential for sustained growth and metastatic spread of cancer, SCLC is more vascularized than non-small cell lung cancer (NSCLC), as shown by a higher microvessel density (8). Patients with SCLC express functional vascular endothelial growth factor receptor (VEGFR)-2 and VEGFR-3 on their tumor cells (9) and have increased levels of serum vascular endothelial growth factor (VEGF) (10). Increased pretreatment levels of VEGF and basic fibroblast growth factor are associated with poor outcome (11-13).
Matrix metalloproteinases
Matrix metalloproteinases (MMPs) are a family of enzymes responsible for remodelling the extracellular matrix in processes of growth and morphogenesis. MMPs and their tissue inhibitors (TIMPs) are important in tumour development and progression. To date, at least 22 related members have been identified. MMPs have been correlated with advanced clinical stage and poor survival in some tumours. The matrix metalloproteinase inhibitors (MMPIs) were among the first agents proposed to act in part via inhibition of angiogenesis to be evaluated in SCLC. Two agents were investigated in randomized trials in SCLC: marimastat (BB 2516, British Biotech) and tanomastat (BAY 12-9566, Bayer Healthcare Pharmaceuticals), the results are disappointing, neither improved survival and side effects adversely impacted on quality of life (14,15).
Thalidomide
Thalidomide is known to possess both immunomodulatory and anti-angiogenic properties, but the mechanism is not well understood. The French Intergroup conducted a randomized phase III trial that suggested a survival advantage (11.7 versus 8.7 months; P=0.16) with the addition of thalidomide as a maintenance therapy versus placebo following response to a four-drug chemotherapy regimen in ED SCLC. Patients with a performance status (PS) of 1 or 2 who received thalidomide had a significantly longer survival (P=0.02) compared to the patients with a PS of 1 or 2 who received the placebo. However, there was a higher incidence of toxicities including thrombosis and neuropathy in the thalidomide arm, which led to about half the patients needing withdrawal or dose reduction (16). The London Lung Cancer Group then conducted a 724-patient randomized phase III trial that evaluated thalidomide versus placebo in combination with carboplatin and etoposide chemotherapy then as maintenance in ED-SCLC. The results show that thalidomide in combination with chemotherapy did not improve survival of patients with SCLC but was associated with an increased risk of thrombotic events. Among patients with limited-stage disease, there was no evidence of a survival difference, but among patients with extensive disease, survival was worse in the thalidomide group. Progression-free survival rates were also similar in the two groups. Thalidomide was associated with an increased risk of having a thrombotic event, mainly pulmonary embolus and deep vein thrombosis (17).
Vascular endothelial growth factor and VEGF receptor (VEGFR)
Bevacizumab
Bevacizumab is a humanized monoclonal antibody targeting the VEGF-A receptor. The promise of antiangiogenic therapy for treatment of solid tumours was first realized with it. This agent has attracted most interest for evaluation in SCLC but its role remains undetermined.
Bevacizumab has been evaluated as a maintenance therapy and in combination with concurrent chemoradiation in LD SCLC.A maintenance phase II study of bevacizumab at a dose of 15 mg/kg after initial concurrent cisplatin, irinotecan and radiotherapy showed good tolerability with objective responses of 80%, 2-year PFS of 54% and a median PFS of 15 months (18). Another single-arm phase II study evaluated maintenance bevacizumab (10 mg/kg) following concurrent irinotecan, carboplatin and radiotherapy in LD SCLC. This study suggested that bevacizumab increases the risk for tracheoesophageal (TE) fistula when administered with and following CRT. Potential mechanisms include enhanced regional tissue injury and impaired mucosal healing (19).
Several phase II trials investigating bevacizumab have been reported in ED SCLC. One trial that that combined bevacizumab with cisplatin and irinotecan in ED SCLC reported an ORR of 75%. Median progression-free survival (PFS) was 7.0 months and median overall survival (OS) was 11.6 months (20). Another phase II trial evaluated carboplatin, irinotecan and bevacizumab in ED SCLC showed an objective response rate (ORR) 84% and median TTP was 9.13 months and median overall survival was 12.1 months. 1- and 2-year overall survivals were 51% and 14%, respectively. Grade 3/4 toxicity (≥10%) included neutropenia (39%), thrombocytopenia (22%), dehydration (10%), diarrhea (31%), fatigue (20%) and pulmonary symptoms (10%). No significant bleeding occurred (21). While these results are promising, patients eligible for bevacizumab are a highly selected population since exclusion criteria for bevacizumab are haemoptysis, presence of brain metastases and hypertension. Results from the randomized phase II SALUTE study, evaluating platinum-etoposide plus bevacizumab versus platinum-etoposide plus placebo in patients with previously untreated ED SCLC, an acceptable toxicity profile, there was a statistically significant improvement in PFS (5.5 versus 4.4 months for bevacizumab arm compared to the placebo arm), the ORR was numerically greater but not statistically significant (58% versus 48%) and there was no improvement in the median OS (9.4 versus 10.9 months) (22).
The Hoosier Oncology group has completed a second-line study of bevacizumab and paclitaxel in patients with sensitive relapsed SCLC, results show that the addition of bevacizumab to paclitaxel does not improve outcomes in relapsed chemosensitive SCLC and none of the vascular endothelial growth factor polymorphisms evaluated were significantly associated with response (23). The Hellenic Oncology Research Group has also comlpeted a second-line study of bevacizumab and paclitaxel in patients with chemoresistant relapsed SCLC, the overall objective response rate was 20%, including one complete remission, whereas the disease control rate was 36.7%, the median progression-free survival 2.7 months and the median overall survival 6.3 months. Grades 3 and 4 toxicities were limited in neutropenia, diarrhea and fatigue. There was one case of non-fatal pulmonary embolism. The results show that the combination of paclitaxel with bevacizumab was feasible and active in this poorognosis and heavily pretreated population of patients with advanced, chemoresistant SCLC, representing a valid therapeutic alternative which merits further evaluation (24).
Cediranib
Cediranib is highly potent inhibitor of VEGFR-1, -2 and -3 tyrosine kinases. A phase II study evaluated its safety and efficacy in relapsed/recurrent SCLC. Of 25 patients recruited, nine patients had stable disease (SD), but none had a confirmed partial response. The median progression-free survival and overall survival were 2 and 6 months, respectively, there was no improvement in the primary endpoint of OS, Cediranib failed to demonstrate objective responses in recurrent or refractory SCLC (25).
Vandetanib
Vandetanib is another multitargeted TKI with dominant activity in vitro against the VEGF receptor. A randomized phase II trial was conducted to investigate vandetanib as a maintenance therapy after complete or partial response following chemotherapy, with or without radiotherapy, in LD and ED SCLC. Vandetanib failed to demonstrate efficacy as maintenance therapy for small-cell lung cancer. The study overall was reported to be negative for any survival benefit but in planned subgroup analyses there was a trend to longer MST in patients with LD SCLC who received vandetanib (26).
Sorafenib and sunitinib
Sorafenib and sunitinib, two other small multitargeted TKIs, are also currently under evaluation in SCLC. Sorafenib is a multiple kinase inhibitor of Raf kinase, VEGFR-2, VEGFR-3 and platelet-derived growth factor receptor (PDGFR) β and affects pathways involved in tumor progression and angiogenesis. A phase II trial was conducted by Southwest Oncology Group, sorafenib was administered at a daily oral total dose of 800 mg to 82 patients with SCLC who had progressed after one platinum-based regimen and patients were stratified by platinum sensitivity. The results show that four partial responses (three in patients sensitive to platinum) and 25 achieved stable disease (12 in patients sensitive to platinum). The median PFS was 2 months, and MST was 6.7 and 5.3 months in the platinum-sensitive and -refractory groups, respectively. Main toxicities included grade 3 skin toxicity in 25% and grade 3/4 flu-like syndrome in 14% of patients (27). Based on the lack of disease control seen in this trial, further investigation of single-agent sorafenib in the small cell lung cancer population is not recommended. Combination trials of sorafenib and chemotherapy are ongoing.
Sunitinib is an oral, small-molecule, multitargeted receptor tyrosine kinase inhibitor active against PDGFR-a and PDGFR-b, VEGFR-1, VEGFR-2 and VEGFR-3, stem cell factor receptor (kit), FMS-like tyrosine kinase 3 (FLT3), colony stimulating factor receptor (CSF-1R) and the glial cell-line derived neurotrophic factor receptor (RET). A phase II study of sunitinib was conducted to evaluate the efficacy and safety in patients with relapsed or refractory SCLC (28). 24 patients received sunitinib (50 mg/day) for four weeks on and two weeks off in a 6-week cycle and 23 were evaluated for response. The ORR was 9% and the median PFS and OS were 1.4 and 5.6 months, respectively. Grade 3/4 toxicity for sunitinib included thrombocytopenia (63%), neutropenia (25%), asthenia (8%) and anorexia (8%). One or two dose reductions were required by 46% of patients. This approach does not appear to warrant further clinical study. Another phase II study evaluated irinotecan and carboplatin followed by maintenance sunitinib in the first-line treatment of ED SCLC. A total of 34 patients were enrolled. The results show that maintenance sunitinib was well tolerated following platinum doublet chemotherapy as first-line treatment for ED SCLC. All patients without progression or intolerable toxicity continued receiving single-agent sunitinib (25 mg orally daily) until progression. The median TTP was 7.6 months and the 6-month ORR was 91%. No grade 3/4 toxicities were observed in the four patients who received sunitinib (29), this phase II trial provides support for further study of sunitinib maintenance therapy following platinum-doublet chemotherapy in patients with ES-SCLC. The 1 year OS of 54% is encouraging, and a randomized trial would be appropriate to assess sunitinib’s impact following chemotherapy. The combination of sunitinib (25 mg/day days 1-14) with standard dose cisplatin and etoposide for untreated ED SCLC appeared to cause prolonged neutropenia and an unacceptable rate of treatment-related mortality. This combination of chemotherapy and sunitinib is not recommended (30).
Aflibercept
Aflibercept (Sanofi-Aventis and Regeneron Pharmaceuticals) is an angiogenesis inhibitor with a unique mechanism of action. It is a fusion protein comprised of segments of the extracellular domains of VEGFR-1 and VEGFR-2 fused to the constant region (Fc) of human IgG1 that functions as a soluble decoy receptor, binding to VEGFA and B, thereby preventing binding to their cell receptors. Topotecan with or without Aflibercept in treating patients with ED SCLC is currently being investigated in a phase II trial.
Targeting cell-signaling pathways controlling proliferation
c-Kit receptor tyrosine kinase
Imatinib is a phenylaminodipyrimidine derivative that targets the tyrosine kinase domain of the hybrid bcr-abl kinase protein as well as c-kit and platelet-derived growth factor receptor (PDGFR). Preclinical findings show that overexpression of c-kit in 28-73% of SCLC, its use in SCLC presented a novel molecular therapeutic approach (31,32).
However, none of four phase II trials of imatinib demonstrated sufficient efficacy in either overall response rate or survival for further development. The first trial administered imatinib by Johnson et al. at a dose of 600 mg once daily (OD) in 19 untreated ED or relapsed sensitive SCLC patients (9 chemonaïve patients with ED and 10 sensitive relapsed SCLC patients). Tumor tissue samples from four (21%) of the 19 patients had the KIT receptor (CD117). There were no objective responses (33). Another phase II trial evaluated high-dose imatinib (up to 400 mg twice daily) in relapsed or treatment-refractory SCLC with proven c-kit overexpression as identified by immunohistochemistry (IHC), but there were no objective responses, and all patients had disease progression by week 4 (34). The trial performed by Dy et al. also evaluated imatinib for patients with relapsed SCLC with c-kit expression and the result was also negative (35). Schneider et al. evaluated maintenance imatinib after treatment with irinotecan and cisplatin chemotherapy for c-kit overexpressing ED SCLC again with no evidence for benefit (36). In combination with irinotecan and cisplatin chemotherapy, imatinib also failed to demonstrate improvement in overall response rate and survival. Moreover, the combination was toxic with an increase in grade 3-4 neutropenia and diarrhoea, possibly due to impaired clearance of irinotecan in the presence of imatinib (37).
Epidermal growth factor receptor tyrosine kinase (EGFR-TK)
Gefitinib is a small molecule EGFR-TKI, it was tested in previously treated chemosensitive SCLC in a small phase II trial. There was no improvement in response rate or survival (38). The low incidence of EGFR exon 19 or 21 mutations (39,40) may be the cause of the negative result of the study.
Erlotinib is another small molecule EGFR-TKI, there is not any reports about its treatment in SCLC.
c-MET receptor tyrosine kinase
The receptor tyrosine kinase c-MET and its ligand, hepatocyte growth factor (HGF), regulate multiple cellular processes that stimulate cell proliferation, invasion and angiogenesis. Mutations in c-MET have been identified in a small proportion of SCLC and c-MET expression is increased at the invasive front in SCLC biopsies. Ma et al. have shown that c-MET/HGF pathway is functional and c-MET is often mutated in SCLC (41). Increased expression of the ligand for c-MET (HGF) is also associated with worse survival. A number of agents have been developed and AMG 102 (Amgen Inc) is currently in trial in SCLC as first-line therapy combined with platinum and etoposide (42,43).
Insulin-like growth factor receptor tyrosine kinase
The insulin-like growth factors (IGF) and their receptors play pivotal roles in cellular signaling transduction and thus regulate cell growth, differentiation, apoptosis, transformation and other important physiological progresses. The insulin-like growth factor 1 receptor (IGF-1R) mainly engages in the Ras/mitogen-activated protein kinase (MAPK) pathway and the phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) pathway, and this pathway has been demonstrated to lower the threshold for chemotherapy-induced apoptosis via activation of the phosphatidylinositol 3-kinase (PI3K)-Akt pathway, as well as promote invasion and metastasis (44). There are already dozens of agents developed for the inhibition of IGF-1R, which are categorized into monoclonal antibodies, small molecule inhibitors and so on. Agents in this class of novel agents under evaluation include AMG 479 (Amgen Inc), IMC-A12 (cixutumumab, ImClone Systems Inc) and CP 571,871 (Pfizer Inc), and AMG 479 is currently in clinical trial in combination with platinum and etoposide chemotherapy in SCLC.
Farnesyltransferase
Farnesyltransferase is an enzyme that is involved in the covalent addition of a farnesyl group to several G-proteins including ras proteins essential for intracellular signal transduction. R115777 (tipifarnib, ZarnestraTM) is a farnesyl transferase inhibitor that blocks the farnesylation of proteins involved in signal transduction pathways critical for cell proliferation and survival. A phase II trial was conducted to evaluated tipifarnib as a monotherapy in patients with sensitive relapsed SCLC. No objective responses were seen, nor was there improvement in progression free survival (PFS) or median OS and so the trial was terminated early (45).
Src kinase
SRC is an oncogene with an essential role in the invasiveness and metastasis of solid tumors including small cell lung cancer. Dasatinib is an oral multikinase inhibitor that inhibits src-family kinases, c-kit, PDGFR-b and bcr-abl proteins. A phase II study of dasatinib was to determine the efficacy of second-line dasatinib in patients with chemosensitive relapsed small cell lung cancer (Cancer and Leukemia Group B 30602). Dasatinib did not reach the efficacy criteria in this clinical setting and the study was terminated (46).
The mammalian target of rapamycin (mTOR)
mTOR signaling pathway senses and integrates a variety of environmental cues to regulate organismal growth and homeostasis. The pathway regulates many major cellular processes and is implicated in an increasing number of pathological conditions including cancer. Two mTOR inhibitors that have been evaluated in SCLC are temsirolimus and everolimus. In one phase II trial that investigated temsirolimus as a maintenance therapy, the PFS was 1.9 months for those patients receiving 25 mg temsirolimus whilst those patients on 250 mg had a PFS of 2.5 months (P=0.24) (47). In another phase II study evaluating everolimus as a maintenance monotherapy following completion of standard chemotherapy in relapsed SCLC, everolimus was well tolerated with only a few CTC grade 3 toxicities and no grade 4 or more toxicities. However, there was no significant improvement in disease control rate (DCR 26%, duration of disease control 2.7-6.3 months), which was the primary endpoint of the study. The median PFS and OS were 1.4 and 5.5 months, respectively (48). The results show that everolimus was well tolerated but had limited single-agent antitumor activity in unselected previously treated patients with relapsed SCLC. Further evaluation in combination regimens for patients with sensitive relapse may be considered. A preliminary report from the other phase II trial showed the similar results (49). Everolimus in combination with chemotherapy in previously untreated patients with SCLC have been undertaken (50). The results worth looking forward to.
Apoptosis promoters
Bcl-2
Bcl2, a powerful anti-apoptotic frequently that is found in high concentrations in SCLC, could possibly be responsible for an increase in chemoresistance, thus targeting Bcl-2 could provide therapeutic benefit (51,52). The results of a phase II study evaluating the addition of an antisense bcl2 oligonucleotide to carboplatin–etoposide as first-line treatment for ED SCLC were disappointing as the addition showed no benefit on OS and survival without relapse and grade 3/4 haematological toxicities were also higher for oblimersen (53). Other novel Bcl-2 family inhibitors, such as obatoclax mesylate and ABT-263 are two BH3 mimetics that have shown promise in preclinical models and are currently undergoing investigation for use in the treatment of SCLC (54). In animal models, ABT-737 induced marked regression and cure of SCLC xenografts (55). AT-101 (gossypol acetic acid, Ascenta Pharmaceuticals), an orally administered bcl-2 inhibitor from the BH3 mimetic family, has been evaluated in the phase I/II setting in combination with topotecan in platinum-pretreated patients with relapsed or refractory SCLC. Though AT-101 appeared safe for administration in conjunction with topotecan and no appreciable difference in the toxicity profile compared to topotecan alone, there was no evident improvement in efficacy with a lack of objective responses and further enrollment to this trial was halted (56).
26S ubiquitin-proteosome complex
Bortezomib is an inhibitor of the 26S ubiquitin-proteosome complex. In preclinical models, bortezomib inhibits the growth of small cell lung cancer by inhibiting the antiapoptotic Bcl-2 signaling pathway (57,58). A phase II trial of bortezomib as a monotherapy in platinum-pretreated relapsed ED SCLC failed to demonstrate efficacy (58). As shown in preclinical models, testing of bortezomib in combination with an apoptotic trigger such as chemotherapy, is a rational clinical approach. A trial of topotecan plus bortezomib has been initiated to test this concept.
Polo-like kinase 1
Polo-like kinase 1 (PLK1) plays key roles in the regulation of mitotic progression, including mitotic entry, spindle formation, chromosome segregation, and cytokinesis. Many studies have shown that high PLK1 expression often correlates with poor prognosis. Using a variety of methods, including small-molecule inhibition of PLK1 function and/or activity, apoptosis in cancer cell lines, cell cycle arrest in normal cell lines, and antitumor activity in vivo have been observed (59). The Plk1 inhibitor BI 2536 was investigated as a monotherapy in relapsed sensitive SCLC in an open-label two-stage phase II trial but after stage I the trial was terminated due to lack of antitumour activity (60).
Histone deacetylase
Histone deacetylase (HDAC) inhibitors have led to tumor growth inhibition and apoptosis in vivo (61,62). There are some trials that are ongoing to investigate various HDAC inhibitors such as vorinostat, belinostat and entinostat. Of all the apoptosis promoting agents currently in development, the spotlight is on the BH3 mimetics as the agents with greatest potential for therapeutic efficacy.
Multidrug resistance inhibitors
Chemotherapy for patients who develop recurrent SCLC is less effective than the initial chemotherapy. A number of resistance mechanisms have been identified in SCLC, including up-regulated expression of genes involved in multidrug resistance (MDR). Biricodar (VX-710, IncelTM, Vertex Pharmaceutical) is a multidrug resistance inhibitor that acts on P-glycoprotein and multi-drug resistance-associated protein-1 (MDR-1), both of which are proteins involved in chemotherapy resistance in cancer. It was studied in a phase II trial in patients with relapsed SCLC in combination with doxorubicin and vincristine. The response rate observed was low (at 19%), and there was a high incidence of grade 3/4 neutropenia (53%) including two mortalities from sepsis. Although there were durable responses, the side effects were serious and the study was terminated early (63).
Vaccines and immune conjugates
p53 cancer vaccine
Cells become susceptible to DNA damage and dysregulated cell growth if p53 genes are deleted or mutated. On this basis, gene therapies targeting p53 have been explored. A trial evaluated the combination of a p53 cancer vaccine with chemotherapy in patients with ED SCLC. This vaccine, consisting of dendritic cells transduced with the full-length wild-type p53 gene, was delivered via an adenoviral vector. A total of 29 patients with ED SCLC were vaccinated repeatedly at 2-week intervals. Most of the patients received three immunizations. p53-specific T cell responses to vaccination were observed in 57.1% of patients. Interestingly, patients who mounted a T-cell response were found to have a higher response rate to second-line chemotherapy compared to those who did not (64).
BB-10901
CD56 is expressed on small-cell lung cancer and other solid tumors of neuroendocrine origin. BB-10901 (IMGN901) consists of a CD56-binding antibody with a potent cancer-cell killing agent, DM1. It acts by delivering the cytotoxic component DM-1 internally via a transmembrane receptor, leading to tubulin polymerization and subsequent cell death. BB-10901 has shown encouraging early activity in patients with pretreated, relapsed or refractory SCLC, with activity demonstrated in second- or greater-line settings and a favourable safety profile (65).
BEC2
BEC2 (mitumomab, ImClone Systems) is an anti-idiotypic murine IgG2b monoclonal antibody that is known to mimic GD3, a ganglioside is expressed on the cell surface of most SCLC cells. BEC2 monoclonal antibody is used in conjunction with bacille Calmette-Guerin (bCG) vaccine to mount an endogenous immune response to GD3. A phase III trial was undertaken to evaluate the use of BEC-2 (2.5 mg)/bCG adjuvant vaccination as a maintenance therapy versus none in patients with LD SCLC who have responded to chemotherapy. A total of 515 patients were randomly assigned. The primary toxicities of vaccination were transient skin ulcerations and mild flu-like symptoms. There was no improvement in survival, PFS or quality of life in the vaccination arm. The OS from randomization was 16.4 and 14.3 months in the observation and vaccination arms (P=0.28), respectively (66).
Discussion
The SCLC is a significant healthcare problem worldwide because of its aggressive nature and high propensity for relapse its aggressive nature and high propensity for relapse. Several targeted agents have altered the paradigm of treatment in some cancer groups. However, we have yet to see a revolution of the same magnitude in the treatment of SCLC (Please see Table 2). The preclinical findings of the various aberrant processes in this type of cancer have not yet been successfully translated into better outcomes with the addition of the novel targeted agents. The low expression or mutation of a gene may have caused the negative results of some clinical trials. We should select suitable SCLC patients in further clinical trials by characterizing the targeted gene and the results of previous clinical trials including the promising results from subgroup analysis.
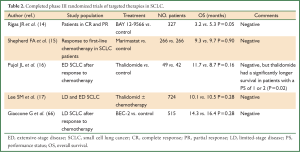
Full Table
Translational research is
In conclusion, to date, no targeted therapy being approved for use in SCLC. Clinical research in this field is still in progress, the results of trials on bevacizumab, gefitinib and bcl-2 inhibtors are promising. With new translational tools, knowledge of SCLC biology and innovative trial designs is increasing, we believe that it should hopefully not be too long before we see a significant therapeutic breakthrough with targeted therapy for this highly aggressive disease.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71-96. [PubMed]
- Gustafsson BI, Kidd M, Chan A, et al. Bronchopulmonary neuroendocrine tumors. Cancer 2008;113:5-21. [PubMed]
- Rudin CM, Hann CL, Peacock CD, et al. Novel systemic therapies for small cell lung cancer. J Natl Compr Canc Netw 2008;6:315-22. [PubMed]
- Jackman DM, Johnson BE. Small-cell lung cancer. Lancet 2005;366:1385-96. [PubMed]
- Turrisi AT 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999;340:265-71. [PubMed]
- Sher T, Dy GK, Adjei AA. Small cell lung cancer. Mayo Clin Proc 2008;83:355-67. [PubMed]
- Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med 2007;357:664-72. [PubMed]
- Lucchi M, Mussi A, Fontanini G, et al. Small cell lung carcinoma (SCLC): the angiogenic phenomenon. Eur J Cardiothorac Surg 2002;21:1105-10. [PubMed]
- Tanno S, Ohsaki Y, Nakanishi K, et al. Human small cell lung cancer cells express functional VEGF receptors, VEGFR-2 and VEGFR-3. Lung Cancer 2004;46:11-9. [PubMed]
- Tas F, Duranyildiz D, Oguz H, et al. Serum vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8) levels in small cell lung cancer. Cancer Invest 2006;24:492-6. [PubMed]
- Salven P, Ruotsalainen T, Mattson K, et al. High pre-treatment serum level of vascular endothelial growth factor (VEGF) is associated with poor outcome in small-cell lung cancer. Int J Cancer 1998;79:144-6. [PubMed]
- Ruotsalainen T, Joensuu H, Mattson K, et al. High pretreatment serum concentration of basic fibroblast growth factor is a predictor of poor prognosis in small cell lung cancer. Cancer Epidemiol Biomarkers Prev 2002;11:1492-5. [PubMed]
- Ueno K, Inoue Y, Kawaguchi T, et al. Increased serum levels of basic fibroblast growth factor in lung cancer patients: relevance to response of therapy and prognosis. Lung Cancer 2001;31:213-9. [PubMed]
- Rigas JR, Denham CA, Rinaldi D, et al. O-107 adjuvant targeted therapy in unresectable lung cancer: the results of two randomized placebo-controlled trials of bay 12-9566, a matrix metalloproteinase inhibitor (MMPI). Lung Cancer 2003;41:S34.
- Shepherd FA, Giaccone G, Seymour L, et al. Prospective, randomized, double-blind, placebo-controlled trial of marimastat after response to first-line chemotherapy in patients with small-cell lung cancer: a trial of the National Cancer Institute of Canada-Clinical Trials Group and the European Organization for Research and Treatment of Cancer. J Clin Oncol 2002;20:4434-9. [PubMed]
- Pujol JL, Breton JL, Gervais R, et al. Phase III double-blind, placebo-controlled study of thalidomide in extensive-disease small-cell lung cancer after response to chemotherapy: an intergroup study FNCLCC cleo04 IFCT 00-01. J Clin Oncol 2007;25:3945-51. [PubMed]
- Lee SM, Woll PJ, Rudd R, et al. Anti-angiogenic therapy using thalidomide combined with chemotherapy in small cell lung cancer: a randomized, double-blind, placebo-controlled trial. J Natl Cancer Inst 2009;101:1049-57. [PubMed]
- Patton JF, Spigel DR, Greco FA, et al. Irinotecan (I), carboplatin (C), and radiotherapy (Rt) followed by maintenance bevacizumab (b) in the treatment (tx) of limited-stage small cell lung cancer (LS-SCLC): update of a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol 2006;24:abstr 7085.
- Spigel DR, Hainsworth JD, Farley C, et al. Tracheoesophageal (Te) fistula development in a phase II trial of concurrent chemoradiation (crt) and bevacizumab (b) in limited-stage small-cell lung cancer (LS-SCLC). J Clin Oncol 2008;26:abstr 7554.
- Ready NE, Dudek AZ, Pang HH, et al. Cisplatin, irinotecan, and bevacizumab for untreated extensive-stage small-cell lung cancer: CALGB 30306, a phase II study. J Clin Oncol 2011;29:4436-41. [PubMed]
- Spigel DR, Greco FA, Zubkus JD, et al. Phase II trial of irinotecan, carboplatin, and bevacizumab in the treatment of patients with extensive-stage small-cell lung cancer. J Thorac Oncol 2009;4:1555-60. [PubMed]
- Spigel DR, Townley PM, Waterhouse DM, et al. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J Clin Oncol 2011;29:2215-22. [PubMed]
- Jalal S, Bedano P, Einhorn L, et al. Paclitaxel plus bevacizumab in patients with chemosensitive relapsed small cell lung cancer: a safety, feasibility, and efficacy study from the Hoosier Oncology Group. J Thorac Oncol 2010;5:2008-11. [PubMed]
- Mountzios G, Emmanouilidis C, Vardakis N, et al. Paclitaxel plus bevacizumab in patients with chemoresistant relapsed small cell lung cancer as salvage treatment: a phase II multicenter study of the Hellenic Oncology Research Group. Lung Cancer 2012;77:146-50. [PubMed]
- Ramalingam SS, Belani CP, Mack PC, et al. Phase II study of Cediranib (AZD 2171), an inhibitor of the vascular endothelial growth factor receptor, for second-line therapy of small cell lung cancer (National Cancer Institute #7097). J Thorac Oncol 2010;5:1279-84. [PubMed]
- Arnold AM, Seymour L, Smylie M, et al. Phase II study of vandetanib or placebo in small-cell lung cancer patients after complete or partial response to induction chemotherapy with or without radiation therapy: National Cancer Institute of Canada Clinical Trials Group Study BR.20. J Clin Oncol 2007;25:4278-84. [PubMed]
- Gitlitz BJ, Moon J, Glisson BS, et al. Sorafenib in platinum-treated patients with extensive stage small cell lung cancer: a Southwest Oncology Group (SWOG 0435) phase II trial. J Thorac Oncol 2010;5:1835-40. [PubMed]
- Han JY, Kim HY, Lim KY, et al. A phase II study of sunitinib in patients with relapsed or refractory small cell lung cancer. Lung Cancer 2013;79:137-42. [PubMed]
- Spigel DR, Greco FA, Rubin MS, et al. Phase II study of maintenance sunitinib following irinotecan and carboplatin as first-line treatment for patients with extensive-stage small-cell lung cancer. Lung Cancer 2012;77:359-64. [PubMed]
- Ready N, Dunphy F, Pang H, et al. Combination chemotherapy with sunitinib (IND 74019; NSC 736511) for untreated extensive-stage small cell lung cancer (SCLC): CALGB 30504 phase IB safety results. J Clin Oncol 2010;28:abstr 7056.
- Krug LM, Crapanzano JP, Azzoli CG, et al. Imatinib mesylate lacks activity in small cell lung carcinoma expressing c-kit protein: a phase II clinical trial. Cancer 2005;103:2128-31. [PubMed]
- Potti A, Moazzam N, Ramar K, et al. CD117 (c-KIT) overexpression in patients with extensive-stage small-cell lung carcinoma. Ann Oncol 2003;14:894-7. [PubMed]
- Johnson BE, Fischer T, Fischer B, et al. Phase II study of imatinib in patients with small cell lung cancer. Clin Cancer Res 2003;9:5880-7. [PubMed]
- Krug LM, Crapanzano JP, Azzoli CG, et al. Imatinib mesylate lacks activity in small cell lung carcinoma expressing c-kit protein: a phase II clinical trial. Cancer 2005;103:2128-31. [PubMed]
- Dy GK, Miller AA, Mandrekar SJ, et al. A phase II trial of imatinib (ST1571) in patients with c-kit expressing relapsed small-cell lung cancer: a CALGB and NCCTG study. Ann Oncol 2005;16:1811-6. [PubMed]
- Schneider BJ, Kalemkerian GP, Ramnath N, et al. Phase II trial of imatinib maintenance therapy after irinotecan and cisplatin in patients with c-Kit-positive, extensive-stage small-cell lung cancer. Clin Lung Cancer 2010;11:223-7. [PubMed]
- Johnson FM, Krug LM, Tran HT, et al. Phase I studies of imatinib mesylate combined with cisplatin and irinotecan in patients with small cell lung carcinoma. Cancer 2006;106:366-74. [PubMed]
- Moore AM, Einhorn LH, Estes D, et al. Gefitinib in patients with chemo-sensitive and chemo-refractory relapsed small cell cancers: a Hoosier Oncology Group phase II trial. Lung Cancer 2006;52:93-7. [PubMed]
- Shiao TH, Chang YL, Yu CJ, et al. Epidermal growth factor receptor mutations in small cell lung cancer: a brief report. J Thorac Oncol 2011;6:195-8. [PubMed]
- Tatematsu A, Shimizu J, Murakami Y, et al. Epidermal growth factor receptor mutations in small cell lung cancer. Clin Cancer Res 2008;14:6092-6. [PubMed]
- Ma PC, Kijima T, Maulik G, et al. c-MET mutational analysis in small cell lung cancer: novel juxtamembrane domain mutations regulating cytoskeletal functions. Cancer Res 2003;63:6272-81. [PubMed]
- Maulik G, Kijima T, Ma PC, et al. Modulation of the c-Met/hepatocyte growth factor pathway in small cell lung cancer. Clin Cancer Res 2002;8:620-7. [PubMed]
- Takigawa N, Segawa Y, Maeda Y, et al. Serum hepatocyte growth factor/scatter factor levels in small cell lung cancer patients. Lung Cancer 1997;17:211-8. [PubMed]
- Warshamana-Greene GS, Litz J, Buchdunger E, et al. The insulin-like growth factor-I (IGF-I) receptor kinase inhibitor NVP-ADW742, in combination with STI571, delineates a spectrum of dependence of small cell lung cancer on IGF-I and stem cell factor signaling. Mol Cancer Ther 2004;3:527-35. [PubMed]
- Heymach JV, Johnson DH, Khuri FR, et al. Phase II study of the farnesyl transferase inhibitor R115777 in patients with sensitive relapse small-cell lung cancer. Ann Oncol 2004;15:1187-93. [PubMed]
- Miller AA, Pang H, Hodgson L, et al. A phase II study of dasatinib in patients with chemosensitive relapsed small cell lung cancer (Cancer and Leukemia Group B 30602). J Thorac Oncol 2010;5:380-4. [PubMed]
- Pandya KJ, Dahlberg S, Hidalgo M, et al. A randomized, phase II trial of two dose levels of temsirolimus (CCI-779) in patients with extensive-stage small-cell lung cancer who have responding or stable disease after induction chemotherapy: a trial of the Eastern Cooperative Oncology Group (E1500). J Thorac Oncol 2007;2:1036-41. [PubMed]
- Tarhini A, Kotsakis A, Gooding W, et al. Phase II study of everolimus (RAD001) in previously treated small cell lung cancer. Clin Cancer Res 2010;16:5900-7. [PubMed]
- Owonikoko TK, Stoller RG, Petro D, et al. Phase II study of Rad001 (everolimus) in previously treated small cell lung cancer (SCLC). J Clin Oncol 2008;26:abstr 19017.
- Besse B, Suk Heist R, Papadimitrakopoulou V, et al. Phase I dose-escalation study investigating Rad001 (R)in combination with cisplatin (C) and etoposide (E) inpreviously untreated patients with extensive-stage disease small-cell lung cancer (ED-SCLC). 13th World Conference on Lung Cancer. San Francisco, California, USA, 2009.
- Yan JJ, Chen FF, Tsai YC, et al. Immunohistochemical detection of Bcl-2 protein in small cell carcinomas. Oncology 1996;53:6-11. [PubMed]
- Jiang SX, Kameya T, Sato Y, et al. Bcl-2 protein expression in lung cancer and close correlation with neuroendocrine differentiation. Am J Pathol 1996;148:837-46. [PubMed]
- Rudin CM, Salgia R, Wang X, et al. Randomized phase II Study of carboplatin and etoposide with or without the bcl-2 antisense oligonucleotide oblimersen for extensive-stage small-cell lung cancer: CALGB 30103. J Clin Oncol 2008;26:870-6. [PubMed]
- Gandhi L, Camidge DR, Ribeiro de Oliveira M, et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol 2011;29:909-16. [PubMed]
- Hann CL, Daniel VC, Sugar EA, et al. Therapeutic efficacy of ABT-737, a selective inhibitor of BCL-2, in small cell lung cancer. Cancer Res 2008;68:2321-8. [PubMed]
- Heist RS, Fain J, Chinnasami B, et al. A phase I/II (P1/P2) study of at-101 in combination with topotecan(t) in patients with relapsed or refractory small cell lung cancer (SCLC) after prior platinum-containing first-line chemotherapy. J Clin Oncol 2009;27:abstr 8106.
- Mortenson MM, Schlieman MG, Virudachalam S, et al. Reduction in BCL-2 levels by 26S proteasome inhibition with bortezomib is associated with induction of apoptosis in small cell lung cancer. Lung Cancer 2005;49:163-70. [PubMed]
- Lara PN Jr, Chansky K, Davies AM, et al. Bortezomib (PS-341) in relapsed or refractory extensive stage small cell lung cancer: a Southwest Oncology Group phase II trial (S0327). J Thorac Oncol 2006;1:996-1001. [PubMed]
- Lansing TJ, McConnell RT, Duckett DR, et al. In vitro biological activity of a novel small-molecule inhibitor of polo-like kinase 1. Mol Cancer Ther 2007;6:450-9. [PubMed]
- Gandhi L, Chu QS, Stephenson J, et al. An open label phase II trial of the Plk1 inhibitor Bi 2536, in patients with sensitive relapse small cell lung cancer (SCLC). J Clin Oncol 2009; 27:abstr 8108.
- Luchenko VL, Salcido CD, Zhang Y, et al. Schedule-dependent synergy of histone deacetylase inhibitors with DNA damaging agents in small cell lung cancer. Cell Cycle 2011;10:3119-28. [PubMed]
- Gray J, Cubitt CL, Zhang S, et al. Combination of HDAC and topoisomerase inhibitors in small cell lung cancer. Cancer Biol Ther 2012;13:614-22. [PubMed]
- Gandhi L, Harding MW, Neubauer M, et al. A phase II study of the safety and efficacy of the multidrug resistance inhibitor VX-710 combined with doxorubicin and vincristine in patients with recurrent small cell lung cancer. Cancer 2007;109:924-32. [PubMed]
- Antonia SJ, Mirza N, Fricke I, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res 2006;12:878-87. [PubMed]
- Fossella F, Woll P, Lorigan P, et al. Clinical Experience of Imgn901 (Bb-10901) in patients with small cell lung carcinoma (SCLC). 13th World Conference on Lung Cancer, San Francisco, 2009.
- Giaccone G, Debruyne C, Felip E, et al. Phase III study of adjuvant vaccination with Bec2/bacille Calmette-Guerin in responding patients with limited-disease small-cell lung cancer (European Organisation for Research and Treatment of Cancer 08971-08971B; Silva Study). J Clin Oncol 2005;23:6854-64. [PubMed]
- Greystoke A, Cummings J, Ward T, et al. Optimisation of circulating biomarkers of cell death for routine clinical use. Ann Oncol 2008;19:990-5. [PubMed]
- Hou JM, Greystoke A, Lancashire L, et al. Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am J Pathol 2009;175:808-16. [PubMed]


