Theobromine for the treatment of persistent cough: a randomised, multicentre, double-blind, placebo-controlled clinical trial
Introduction
At any one time, 10% of the population have a persistent cough (1) and acute cough is the most common reason for new patient consultation in primary care. Sufferers consume 75 million doses of over-the-counter antitussive medication annually, totalling £98.7 million in the UK in 2014 (2,3). Codeine and dextromethorphan are the most commonly prescribed opioid-derived antitussives but their use is limited by unpredictable efficacy and unacceptable adverse events (AEs), such as somnolence, dizziness, nausea and constipation (4-7).
Theobromine is a methylxanthine alkaloid derivative that is predominant in cocoa (8,9). It has a long history of clinical use and has been suggested as a promising therapy for the treatment of persistent cough (4,10-12). BC1036 is a non-opioid, non-narcotic, theobromine treatment and its main mechanisms of action are inhibition of phosphodiesterases and blockade of adenosine receptors. Theobromine has been shown to inhibit the inappropriate firing of the vagus nerve, which is a key feature of persistent cough. This peripheral mechanism of action differentiates theobromine from codeine and other centrally acting agents, and lessens its lower central nervous system AEs. Theobromine has been approved since 2009 in Korea for persistent cough under the name AnyCoughTM (developed by Ahn-Gook Pharmaceuticals, Seoul, South Korea). This study was carried out to assess the effectiveness of BC1036 for persistent cough in a randomised, controlled clinical trial setting. The primary objective was to investigate the effect of BC1036 on quality of life (QOL) in subjects with persistent cough. Secondary objectives were to investigate the effect of BC1036 on cough severity and objective cough reflex sensitivity.
Methods
Study design
This was a Phase III, randomised, multicentre, double-blind, placebo-controlled, parallel-group study conducted in 13 UK cough clinics and primary and secondary care centres between 13 July 2012 and 6 August 2013.
Subjects
Main inclusion criteria comprised males or females aged 18–75 years with a confirmed diagnosis of persistent cough. This was defined as a chronic cough lasting ≥8 weeks at baseline or a sub-acute cough lasting ≥3 weeks at baseline. Subjects were required to have a Leicester Cough Questionnaire (LCQ)2 score of ≤17 at baseline and forced expiratory volume in 1 second (FEV1) ≥70% of predicted normal at screening. Key exclusion criteria were an uncontrolled intercurrent illness, treatment with systemic oral steroids, theophylline or theophylline-like agents, or opiates or opioids within 7 days prior to randomization, significant sputum production on any 3 days in the screening period, or current smokers or past smokers who had a smoking history of >20 pack years. In addition, patients were excluded if any of the following applied:
- Any pulmonary abnormality on chest X-ray or computed tomography scan performed in the 12 months prior to enrolment indicative of chronic obstructive pulmonary disorder, bronchiectasis etc.;
- Subjects diagnosed with asthma who had suffered an exacerbation requiring hospitalisation within 4 weeks prior to screening;
- Any pulmonary co-morbidity such as chronic obstructive pulmonary disorder, recurrent lower respiratory tract infections (≥2 within 12 months of screening) and bronchiectasis where cough suppression could have led to sputum retention and infection;
- Pregnant or lactating females.
With respect to all current stable regimens of over-the-counter cough and cold remedies including antihistamines and decongestants, no regimen adjustments and new treatments could be initiated between Visit 1 and Visit 5.
Randomization
Eligible subjects were randomised to receive either BC1036 or placebo in a 1:1 ratio using RANCODE (version 3.6).
Study medication
Subjects were randomised to one of the following treatments, which were blinded by using the same capsules:
- BC1036 capsules: 300 mg of theobromine per capsule, taken orally twice daily (morning and evening), approximately 12 hours apart, for 14 consecutive days. The dose selected was based on the pre- and post-marketing experience of theobromine 300 mg capsules in Korea.
- Matching placebo capsules, taken orally twice daily (morning and evening), approximately 12 hours apart, for 14 consecutive days.
Compliance was calculated as: (Actual number of capsules used/prescribed number of capsules to be taken) ×100. A calculated compliance of <90% or >110% was classified as a major deviation.
Study schedule
Subjects attended the clinic on up to six occasions for consent, screening, baseline, Day 7 (±1 day), Day 14 (±1 day), and a follow-up visit at Day 28 (±2 days). At each visit subjects completed the LCQ, which is a validated, self-reported 19-item questionnaire with a 7-point Likert response scale for each question. The LCQ assesses the impact of cough on QOL over the previous 14 days. In addition to the LCQ, a 100 mm cough visual analogue scale (VAS) was recorded at each visit, using a scale of 0= no cough to 100= worst cough ever. Vital signs (pulse rate, blood pressure and body temperature) and pulmonary function were also measured at each visit. Blood and urine samples were collected for safety clinical laboratory parameters at screening and Day 28, and electrocardiograms (ECGs) were recorded at screening and Day 14.
Efficacy endpoints
The primary endpoint comprised cough-related QOL, assessed using the LCQ, with baseline-adjusted total LCQ score at Day 14 being the primary endpoint. The lower the total score, the greater the impact of the cough on various aspects of daily life (2).
Secondary endpoints comprised the baseline-adjusted LCQ scores at Day 7 and Day 28, the cough VAS scores at each visit, and pulmonary function tests at each visit [FEV1, forced vital capacity (FVC) and peak expiratory flow (PEF) measured using a calibrated spirometer].
Ethics and trial registration
This study was performed in accordance with the Declaration of Helsinki. This human study was approved by National Research Ethics Services Committee North East- Sunderland (approval: 11/NE/0375). All adult participants provided written informed consent to participate in this study. The clinical trial was registered on ClinicalTrials.gov with the registration number of NCT01656668.
Sample size justification and statistical analyses
A mean difference between BC1036 and placebo baseline-adjusted total LCQ score of 1.3 (SD 3.2) was considered clinically significant (13). Based on this assumption, for a two-sample pooled t-test of a normal mean difference with a two-sided significance level of 0.05, a sample size of 129 subjects per group was required to obtain a power of at least 0.9 to detect a mean difference of 1.3. Assuming a drop-out rate of 10%, this yielded a total sample size of 144 subjects per treatment, i.e., 288 subjects in total.
The primary efficacy endpoint (cough-related QOL assessed using the LCQ) was analysed by means of an analysis of variance (ANOVA), including treatment and centre effects. Two-sided 95% confidence intervals (CIs) for the difference of baseline-adjusted means between treatment groups were calculated. Missing values for the LCQ score were substituted with the last observation carried forward (LOCF). For all other secondary variables, baseline-adjusted scores were analysed for Days 7, 21 and 28 using descriptive statistics.
All safety data obtained in this study were tabulated with descriptive statistics or counts and percentages depending on the variable.
Results
Subject demographics
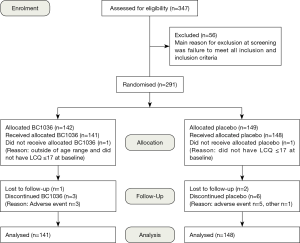
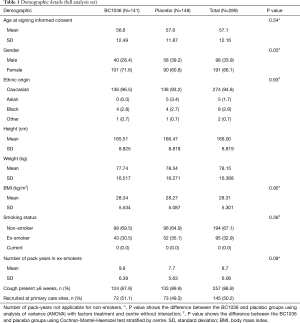
Of the 347 subjects screened, 291 were randomised at 13 UK centres (Figure 1). Subjects had a mean age of 57 years (range, 19–78 years) and a mean body mass index (BMI) of 28.3±5.3 kg/m2. Females outnumbered males by 2:1 (191 females, 98 males) and more females were randomised to BC1036 (71.6% female, 28.4% male) compared to placebo (60.8% female, 39.2% male). There were no statistically significant differences between the treatment groups at baseline with respect to age, gender, ethnic origin, weight, height, BMI, or smoking history (Table 1).
Full table
Exposure to treatment
The study drug or placebo was administered to 289 subjects. Mean compliance was 98.3% for BC1036 and 97.5% for placebo. The mean duration of treatment was 14 days in both groups (range 12–21 days in the BC1036 group and 7–28 days in the placebo group).
Efficacy
Leicester cough questionnaire
The two groups were not matched for QOL at study entry. Mean total LCQ score at baseline in the BC1036 group was significantly lower than for placebo (i.e., had a worse QOL) (10.5±3.4 for BC1039 compared to 12.1±3.0 for placebo; P<0.001), indicating the treatment groups were not homogenous at baseline.
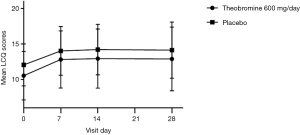
At all assessment points during treatment and follow-up (e.g., at days 7, 14 and 28), the mean baseline-adjusted score was greater for BC1036 compared to placebo, indicating a greater improvement in QOL compared to placebo treatment, but the difference between the groups did not reach statistical significance at any timepoint. At the primary endpoint of Day 14, the mean baseline-adjusted score LCQ was 2.4±3.5 for BC1036 and 2.2±3.0 for placebo (P=0.60; 95% CI: −0.98 to 0.48) (Figure 2). However, for both groups, mean scores were still in the moderately impaired range on the LCQ.
To further investigate the significant difference between the treatment groups at baseline, post-hoc analyses were conducted in a variety of subgroups of subjects to try to identify whether duration of cough, the LCQ score, or time between screening and the start of treatment, or a combination of these factors, could explain this difference. A significant treatment effect was seen in the following 4 subgroups at Day 7, but in all 4 groups the baseline difference between treatment arms was still apparent:
- Subjects with an interval of >7 days between screening and Day 0 (7 days was used as the cut-off value because this was the washout period for prior medications before entry into the study);
- Subjects with an interval of >7 days between screening and Day 0 and baseline LCQ ≤14 (the LCQ of 14 was used since this is corresponds to a moderate cough);
- Subjects with an interval of >7 days between screening and Day 0 and cough ≥8 weeks;
- Subjects with an interval of >7 days between screening and Day 0 and cough ≥8 weeks and baseline LCQ ≤14.
At Day 14, no significant treatment effect was seen for any of the subgroups. Thus, no evidence was found in the post-hoc analyses explaining the significant baseline difference in LCQ between BC1036 and placebo.
Cough VAS
Subjects in the BC1036 group assessed their cough as more severe at baseline compared to the placebo group, although the difference was not statistically significant (mean of 57.5±20.0 for BC1039 compared to 53.1±20.6 for placebo; P=0.066). At each timepoint during the study and at follow-up, a greater reduction in cough severity was seen in the BC1036 group compared to the placebo group, but again the difference was not statistically significant (Table 2).

Full table
Pulmonary function tests
At baseline, the pulmonary function tests were similar in the two groups: mean FEV1 was 2.7±0.8 L for BC1036 and 2.6±0.7 L for placebo, mean FEV1 predicted (%) was 99.0%±99.7% for BC1036 and 97.1%±96.4% for placebo, and mean FVC was 3.4±1.0 L for BC1036 and 3.4±0.8 L for placebo. There were no significant changes in lung function during the study period.
Safety
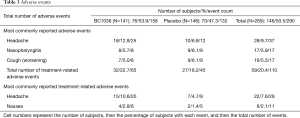
During the study, 50.5% of subjects experienced 290 AEs (53.9% BC1036, 47.3% placebo), the majority of which were mild or moderate intensity. Of these, a total of 111 AEs in 59 subjects were considered drug-related (66 BC1036, 45 placebo) (Table 3).
Full table
Discussion
There are inherent difficulties in conducting cough studies. Cough is a unique symptom which can be under voluntary control (14). Marked heterogeneity in therapeutic response may be seen, dependent on disease type and severity (15). There is a significant placebo effect (up to 85%) associated with over-the-counter cough medicines (14), with this being due to natural recovery, measures of cough severity declining over the course of the trial (regression of cough response toward the mean), the demulcent effect of treatment, and the effect of the sweetness of the cough syrup in soothing cough (14). This is further enhanced by expectation of effect related to advertising, brand, packaging and formulation (14).
There are a number of methods used to measure cough frequency, intensity and severity, and reporting of cough is notoriously unreliable. Subjective measures of cough introduce considerable bias as the outcome relies on individual perception, which can be affected by environmental and psychological factors (16). Although cough scores using diary cards and VASs are widely used as an outcome measure, they are generally inaccurate because they rely on the motivation and vigilance of the subject, and do not consistently correlate with objective methods, such as ambulatory cough monitoring (17). Objective measurement of cough frequency using ambulatory electronic monitors is currently considered to provide greater insight into the reduction of cough frequency (18). At the time this study was conceived, no viable/validated options were available. However, the development of equipment to achieve this has been the subject of much research in recent years. Recent software development has led to significant progress in the development of automated cough detection which largely overcomes the issues of what constitutes “a cough” for the purposes of counting. This should certainly be a consideration for future studies.
Theobromine has been suggested as a promising therapy for treatment of persistent cough. It has a rapid onset, long duration of activity (>4 hours), is substantially more effective than caffeine in inhibiting cough induced by citric acid in the guinea-pig model, and it is at least as effective as theophylline and doxophylline (4). Theobromine has also been shown to suppress capsaicin-induced cough in humans with no AEs, and is considered a novel and promising treatment which may pave the way for a new class of antitussive drugs (4).
This double-blind, placebo-controlled, parallel-group study investigated the effect of BC1036 (theobromine) on the QOL of subjects with persistent cough, with the primary efficacy endpoint being the change from baseline in LCQ score on Day 14. The results showed a greater effect on QOL following BC1036 treatment compared to placebo, but the difference was small and did not reach statistical significance and also did not meet the pre-determined limit for clinical significance that was set at a total LCQ score of 1.3 in the sample size calculation (13). However, for both groups, mean scores were still in the moderately impaired range on the LCQ. For the cough severity score, a greater reduction in cough severity during the study was seen in the BC1036 group compared to the placebo group, but again the difference was small and not statistically significant. However, a confounding factor impacting these results was the finding that the treatment groups were not balanced at baseline, with subjects in the BC1036 group having a more severe persistent cough that had a greater impact on QOL at baseline compared to subjects in the placebo group. Although the statistical analysis was adjusted for baseline severity, the difference was too great to be corrected by modelling alone and thus was thought to impact on the overall results. Despite a post-hoc subgroup analysis, no reason was discovered for this baseline difference. Thus, for future studies, stratification at randomization for cough severity might be considered.
The results of this study highlight the difficulties of conducting cough studies. However, it should be noted that this was the first multicentre trial conducted in persistent cough and the largest trial in cough at the time it was conducted, and much has been learnt from this trial to guide subsequent studies. As improvements in understanding cough hypersensitivity are beginning to influence development of new cough preparations that are effective on all cough types (19), so it is important that future studies assessing cough use validated methods that are sensitive to change. Future trials must be conducted for an appropriate length of time, and details on extent of effects, adherence, and AEs should be reported using clinically relevant outcome measures, including cough frequency, severity and duration (20). One consideration for future trials could be to use composite endpoints which can bring about statistical precision and encompass the several different facets of cough assessment (21). However, any composite endpoint should not be too broad, thus rendering the results clinically meaningless (22). The dose may also have been a factor, as it was based on pre- and post-marketing experience of theobromine 300 mg capsules (Anycough™) which is approved in Korea. Further investigation is needed with regards to the potential for genetic polymorphisms of the metabolising enzymes and other factors that could influence drug metabolism.
In conclusion, this study showed that BC1036 was well tolerated and, although the primary endpoint did not achieve statistical significance, BC1036 showed a small but positive effect on improving subjects’ QOL and also in reducing cough severity.
Acknowledgements
In addition to the primary authors, the following investigators participated in the conduct of this study: Dr. Alun George, The Staploe Medical Centre, Ely; Dr. Alan Jackson, Sheepcot Medical Centre, Watford; Dr. Warwick Coulson, Albany House Medical Centre, Wellingborough, Northampton; Site 9: Dr. Richard Oliver, Ecclesfield Group Practice, Sheffield; Site 10: Dr. Chris Strang, Mortimer Surgery, Reading; Dr. Philip Marazzi, The Medical Centre, Leatherhead; Dr. Damien McNally, Ormeau Health Centre, Belfast. Medical writing support was provided by Debbie Jordan.
This work was supported by Infirst Healthcare Ltd.
Footnote
Conflicts of Interest: AH Morice was chief investigator of this study. He has received speaker’s honoraria from Astra Zeneca, Chiesi, Boehringer Ingelheim, Almirall, and Novartis. He has received sponsorship to attend international scientific meetings from Boehringer Ingelheim, Astra Zeneca and Chiesi. L McGarvey was an investigator in this study, which was funded by Infirst Healthcare. ID Pavord has received speaker’s honoraria in the last 5 years for speaking at sponsored meetings from Astra Zeneca, Boehringer Ingelheim, Aerocrine, Almirall, Novartis, and GSK and payment for organising an educational event from Astra Zeneca. He has received honoraria for attending advisory panels with Almirall, Genentech, Regeneron, Astra Zeneca, Boehringer Ingelheim, GSK, MSD, Schering-Plough, Novartis, Dey, Napp and Respivert. He has received sponsorship to attend international scientific meetings from Boehringer Ingelheim, GSK, Astra Zeneca and Napp. B Higgins was an investigator in this study, which was funded by Infirst Healthcare. KF Chung was an investigator in this study, which was funded by Infirst Healthcare. SS Birring has received personal fees from Infirst Ltd for scientific advisory boards and advice.
Ethical Statement: This study was performed in accordance with the Declaration of Helsinki. This human study was approved by National Research Ethics Services Committee North East - Sunderland (approval: 11/NE/0375). All adult participants provided written informed consent to participate in this study.
References
- Song WJ, Chang YS, Faruqi S, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J 2015;45:1479-81. [Crossref] [PubMed]
- Birring SS, Prudon B, Carr AJ, et al. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 2003;58:339-43. [Crossref] [PubMed]
- Sales value of over-the-counter (OTC) cough/cold/sore throat medicines in Great Britain in 2014, by category (in millions GBP) Available online: , accessed electronic materials: 19 March 2016.http://www.statista.com/statistics/415982/over-the-counter-sales-for-cough-cold-sore-throat-in-great-britain/
- Usmani OS, Belvisi MG, Patel HJ, et al. Theobromine inhibits sensory nerve activation and cough. FASEB J 2005;19:231-3. [PubMed]
- Pavesi L, Subburaj S, Porter-Shaw K. Application and validation of a computerized cough acquisition system for objective monitoring of acute cough: a meta analysis. Chest 2001;120:1121-8. [Crossref] [PubMed]
- Resine T, Pasternak G. Opioid analgesics and antagonists. In: Gilman AG, Goodman LS, Gilman A. editors. The Pharmacological Basis of Therapeutics, 6th edition. New York: McGraw Hill, 1996:521-55.
- Matthys H, Bleicher B, Bleicher U. Dextromethorphan and codeine: objective assessment of antitussive activity in patients with chronic cough. J Int Med Res 1983;11:92-100. [Crossref] [PubMed]
- Graham HN. Tea: the plant and its manufacture; chemistry and consumption of the beverage. Prog Clin Biol Res 1984;158:29-74. [PubMed]
- Shively CA, Tarka SM Jr. Methylxanthine composition and consumption patterns of cocoa and chocolate products. Prog Clin Biol Res 1984;158:149-78. [PubMed]
- Stavric B. Methylxanthines: toxicity to humans. 3. Theobromine, paraxanthine and the combined effects of methylxanthines. Food Chem Toxicol 1988;26:725-33. [Crossref] [PubMed]
- Saano V, Minker E, Joki S, et al. Influence of chinoin-170, a novel antitussive, on the mucociliary activity in respiratory airways of rats, rabbits, guinea-pigs and man. J Pharm Pharmacol 1993;45:799-802. [Crossref] [PubMed]
- Mikus EG, Révész J, Minker E, et al. Experimental studies on the antitussive properties of the new xanthine derivative 1Hpurine-2,6-dione, 3,7-dihydro-3-methyl-7[(5-methyl-1,2,4-oxadiazol-3-yl)methyl]. 1st communication: in vivo demonstration of the effects on animal models of cough and of mucociliary clearance. Arzneimittelforschung 1997;47:395-400. [PubMed]
- Raj AA, Pavord DI, Birring SS. Clinical Cough IV: What is the minimal important difference for the Leicester Cough Questionnaire? In: Chung KJ, Widdicombe J. editors. Pharmacology and Therapeutics of Cough. Handbook of Experimental Pharmacology 187. Berlin Heidelberg: Springer-Verlag, 2008:311-20.
- Eccles R. Importance of placebo effect in cough clinical trials. Lung 2010;188:S53-61. [Crossref] [PubMed]
- Dicpinigaitis PV, Morice AH, Birring SS, et al. Antitussive Drugs - Past, Present, and Future. Pharmacol Rev 2014;66:468-512. [Crossref] [PubMed]
- Dales RE, Spitzer WO, Schechter MT, et al. The influence of psychological status on respiratory symptom reporting. Am Rev Respir Dis 1989;139:1459-63. [Crossref] [PubMed]
- Chang AB. Isolated cough: probably not asthma. Arch Dis Child 1999;80:211-3. [Crossref] [PubMed]
- Leconte S, Liistro G, Lebecque P, et al. The objective assessment of cough frequency: accuracy of the LR102 device. Cough 2011;7:11. [Crossref] [PubMed]
- Morice AH. It's time to change the way we approach coughs in community pharmacy. Pharm J 2016.9.
- Smith SM, Schroeder K, Fahey T. Over-the-counter (OTC) medications for acute cough in children and adults in ambulatory settings. Cochrane Database Syst Rev 2012;8:CD001831. [PubMed]
- Faruqi S, Thompson R, Wright C, et al. Quantifying chronic cough: Objective versus subjective measurements. Respirology 2011;16:314-20. [Crossref] [PubMed]
- Kleist P. Composite endpoints: proceed with caution. Applied Clinical Trials. 1 May 2006. Available online: , accessed electronic materials: 27 April 2016.www.appliedclinicaltrialsonline.com