Benign superior vena cava syndrome with uncontrolled pleural effusion by calcified mediastinal lymphadenopathy: surgical management
Introduction
Superior vena cava syndrome (SVCS) is a medical emergency requiring prompt treatment regardless of the causes. Malignancies account for up to 80% of SVCS cases (1,2). However, a non-malignant condition or some kinds of bacterial infections account for 15–40% of cases in retrospective studies, and thrombosis related to an indwelling intravascular device accounts for a considerable proportion (3,4). Among the benign cases of SVCS, up to 50% is caused by mediastinal fibrosis (5). And, SVCS by anthraco-mediastinal lymphadenopathy is rare (6). So, we report a case of SVCS due to benign anthracofibrotic mediastinal lymphadenopathy accompanying recalcitrant pleural effusions corrected by a bypass graft.
Case presentation
An 81-year-old female was admitted to the emergency room because of uncontrolled pleural effusion and dyspnea for several months. She was treated with asthma and hypertension more than ten years. The treatment started with anti-tuberculous drug (isoniazid 300 mg, rifampin 450 mg, ethambutol 1,200 mg, pyrazinamide 1,000 mg) empirically based on the bronchoscopic findings of anthracotic pigments without any microbiological confirmation in previous hospital.
However, the pleural effusion was not controlled despite administration of anti-tuberculous drug. A chest computed tomography (CT) scan taken at the previous hospital showed right pleural effusion without any evidence of lymphadenopathy or SVC compression. The pleural fluid analysis from right side revealed lymphocyte-dominant (75%), but the cellular composition in repeated aspirations changed from lymphocyte-dominant to neutrophil-dominant (81%). The components of right side pleural fluid were exudate and not consistent with the findings of tuberculosis or malignancy (serum protein 6.3 g/dL, pleural fluid: protein 3.8 g/dL, glucose 131 mg/dL, ADA 19.7 U/L, CEA 1.7 ng/µL). Left side pleural effusion showed also same exudate components similar with right side pleural effusion.
The bilateral pleural effusion did not improve despite of continuous drainage by intercostal chest tube and antibiotic treatments. Severe dyspnea and facial swelling developed about one month after admission. Echocardiographic findings showed normal ejection fraction (68%) with normal systolic function. Retrospective review of Chest CT taken at the day of admission showed a very small low density nodule partially compressing the superior vena cava (SVC). Unfortunately, the radiologist and physician missed to notice the small low density around SVC due to absence of symptoms and signs of SVCS (Figure 1A,B).
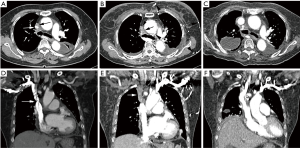
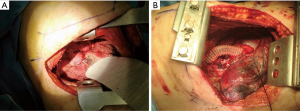
A follow-up chest CT at one month later revealed that an approximately 1.7 cm mass encasing the SVC above the azygous arch was completely obstructing the SVC. The segment of SVC above the mass and both brachiocephalic veins were not enhanced, but multiple collateral veins (Figure 1, arrowheads) were enhanced in the mediastinum, and diffuse skin thickening and edema were noted in the anterior chest wall (Figure 1C,D). The possibility of malignancy of mass encasing SVC was less likely because the mass encasing the SVC appeared within a month which is relatively short periods to suspect the malignancy. A surgical exploration via a right thoracotomy was performed because of rapid aggravation of SVCS although she was a high risk patient for a surgical operation due to severe asthma. Anthracotic calcified lymph nodes tightly were compressing the SVC at the level of the azygous venous branches in operative fields. Because the fibrotic adhesions around the SVC were very severe, it was impossible to dissect the lymph nodes and remove the mass around the SVC. So, a bypass graft was performed using an artificial vessel instead of removing the mass encasing the SVC (Figure 2). After surgery, the SVCS and persistent pleural effusion dramatically disappeared. A chest CT scan after surgery showed patent SVC and brachiocephalic veins with bypass graft (Figure 1E,F). The excised lymph node and adjacent tissue showed diffuse histiocytic infiltration with heavy anthracotic pigmentation and dense collagenous fibrosis, extending to the perinodal soft tissue. The pathological findings revealed no evidence of malignancy or active infection. The anthracotic pigmentation can be explained by the patient’s history of being exposed to biomass fuel such and coal dust, wood for more than 30 years of life long time. This case is characterized by recalcitrant pleural effusions without typical signs of SVCS, and later revealed as anthracotic calcified lymph nodes circumferential compressing the SVC which was successfully treated by surgical bypass graft.
Discussion
SVC obstruction can cause increased hydrostatic venous pressures in turn leading to restriction of the capacity of the lymphatics in the parietal pleura finally resulting in accumulation of pleural fluid. In this case, recalcitrant pleural effusions in presenting case could be explained by progressive SVC obstruction due to encasing anthracofibrotic lymphadenopathy. Histopathologic findings and tissue culture test revealed no evidences of malignancy or active infection. The benign SVCS by anthracofibrotic calcified lymphadenopathy might be caused by long term exposure to the biomass fuels. The elderly women lived in rural areas more than 30 years generally use the biomass fuel such as woods and coal dust for cooking and heating, which frequently leads to development of anthracofibrosis.
Some cases of mediastinal lymphadenopathy accompanying anthracosis without continuous pleural effusion complicated with SVCS were reported by other authors (7,8).
But our case is unique in that recalcitrant pleural effusions and rapid appearance of SVCS by encasing anthracotic lymphadenopathy was presented. A chest CT scan taken after one month revealed total obstruction of SVC by a mass-like lesion which was not observed on CT scan done at admission. Mediastinal fibrosis generally progresses slowly over the years (9), but the rapidly progressing feature in this case does not consistent with the general characteristics of typical mediastinal fibrosis. Mediastinal fibrosis mostly presents as a localized pattern in anterior mediastinum rather than diffuse mediastinal involvement. Our case was atypical in that it was benign SVCS caused by the rare mediastinal fibrosis pattern with circumferential compression surrounding the SVC.
In general benign SVCS can be treated by medical or endovascular treatment such as SVC stenting. But in our case, instead of endovascular stenting surgical bypass graft was performed due to severe circumferential stenosis of SVC with extensive adhesion.
Conclusions
Benign anthracotic calcified lymphadenopathy compressing the SVC should be suspected in a patient with abrupt development of SVCS with recalcitrant pleural effusions without radiological findings of malignancy and for confirmative diagnosis, serial chest CT scans should be recommended.
And surgical decompression of encasing mass around SVC should be considered as a curative modality although the patient is in high risk condition for operation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: This study was approved by the Institutional Review Board of the Ewha Womans University Mokdong Hospital (approval No. EUMC 2014-10-014-002). Written informed consent was obtained from the patient to publish this manuscript and any accompanying images.
References
- Chen JC, Bongard F, Klein SR. A contemporary perspective on superior vena cava syndrome. Am J Surg 1990;160:207-11. [Crossref] [PubMed]
- Parish JM, Marschke RF Jr, Dines DE, et al. Etiologic considerations in superior vena cava syndrome. Mayo Clin Proc 1981;56:407-13. [PubMed]
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006;85:37-42. [Crossref] [PubMed]
- Rozmus G, Daubert JP, Huang DT, et al. Venous thrombosis and stenosis after implantation of pacemakers and defibrillators. J Interv Card Electrophysiol 2005;13:9-19. [Crossref] [PubMed]
- Lepper PM, Ott SR, Hoppe H, et al. Superior vena cava syndrome in thoracic malignancies. Respir Care 2011;56:653-66. [Crossref] [PubMed]
- Chow BJ, McKim DA, Shennib H, et al. Superior vena cava obstruction secondary to mediastinal lymphadenopathy in a patient with cystic fibrosis. Chest 1997;112:1438-41. [Crossref] [PubMed]
- Bilici A, Erdem T, Boysan SN, et al. A case of anthracosis presenting with mediastinal lymph nodes mimicking tuberculous lymphadenitis or malignancy. Eur J Intern Med 2003;14:444-6. [Crossref] [PubMed]
- Onitilo AA, Engel JM, Tanimu SB, et al. Anthracosis and large mediastinal mass in a patient with healed pulmonary tuberculosis. Clin Med Res 2010;8:99-103. [Crossref] [PubMed]
- Novella Sánchez L, Sanz Herrero F, Berraondo Fraile J, et al. Mediastinal fibrosis and superior vena cava syndrome. Arch Bronconeumol 2013;49:340-2. [PubMed]


