Profuse and acute hemorrhagic gastroesophageal injury after cryotherapy in a cardiac surgery patient
Introduction
Upper gastrointestinal injury associated with cryoablation during cardiac surgery is a rare complication but potentially fatal. During cryoablation to treat atrial fibrillation, delivered cryoenergy can adversely affect the esophagus, resulting in gastroesophageal ulceration.
In this case report, we describe a gastroesophageal injury with massive bleeding that occurred by manipulation of an inserted transesophageal echocardiography (TEE) probe during cryoablation therapy in a cardiac surgery patient.
Case presentation
An 88-year-old male (69 kg, 176 cm) presented in the operating room for the replacement of aortic valve with maze operation. On admission, he had exertional dyspnea (New York Heart Association class III) and diagnosed as symptomatic aortic regurgitation (AR) with atrial fibrillation. He had no medical or surgical history except the current disease. He denied any chest pain, difficulty in swallowing, dysphagia, history of gastroesophageal reflux disease, or gastrointestinal bleeding. Physical examination revealed diastolic murmur with irregular heartbeat. Preanesthetic chest X-ray showed fibrocalcified old pulmonary tuberculosis on bilateral upper lobe. Preoperative echocardiography confirmed moderate eccentric AR due to annuloaortic ectasia. It also revealed dilated left ventricle (LV) and both atria, normal LV contractility with moderate tricuspid regurgitation, no regional wall motion abnormality and borderline resting pulmonary hypertension. Atrial fibrillation was found on electrocardiography. The results of the preoperative laboratory investigation were not remarkable: white blood cell count, 3.84×103/µL, red blood cell count, 4.42×106/µL, hemoglobin, 13.3 g/dL, platelet 123×103 /µL, prothrombin time/activated partial thromboplastin time/international normalized ratio, 12.0 seconds/34.5 seconds/1.10, alanine aminotransferase level, 17 IU/L, aspartate aminotransferase level, 28 IU/L, blood urea nitrogen, 15.8 mg/dL, creatinine, 1.20 mg/dL.
He was premedicated with 0.2 mg of glycopyrrolate 30 minutes before arriving in the operating room. After placing an arterial line in the left radial artery, 5 mg of midazolam and 50 mg of rocuronium was administered for the induction of anesthesia under bispectral index monitoring.
Anesthesia was maintained with sevoflurane and remifentanil after tracheal intubation. Continuous cardiac output monitoring was conducted through Swan-Ganz catheter attached to a Vigilance system (Edward Lifescience, USA). The TEE probe (TE-V5Ms, Siemens medical solutions, USA) was advanced to the esophagus and to the stomach without any resistance. The initial TEE revealed moderate to severe AR due to annuloaortic ectasia and prolapse of right coronary cusp with ejection fraction (EF) =55%. The baseline activated clotting time (ACT) was monitored as 155 seconds. We performed a thromboelastography (TEG) immediately after the induction of anesthesia and no abnormality was found on TEG (R, 7.1 minutes, K, 1.4 minutes, α angle 61.4 degree, maximum amplitude, 57.6 mm, LY 30, 0%).
Twenty thousand units of heparin were injected and ACT was checked over 400 seconds. Cardiopulmonary bypass (CPB) was started. Aortic valve replacement using prosthetic tissue valve was conducted and maze operation with cryoablation was performed. All lesions were created by applying the CryoMaze Probe (ATS Medical, Minnesota, USA) for 2 minutes directly to myocardial tissue with temperatures reaching –70 °C. Following lesion creation, the probe was thawed from the surrounding tissue by administering cold saline for the left-sided lesions and room temperature saline for the right-sided lesions. The left-sided lesions were created under cardiac standstill and the right-sided lesions were created on the beating heart, while rewarming. Left atrial cryoablation was done by pulmonary vein isolation, left atrial isthmus lesion, left atrial appendage lesion and coronary sinus lesion. Interatrial septal lesion with two endocardial lesions was created for right side.
During the cryoablation of left side with arrested heart, we recognized that the TEE probe had not been withdrawn before the cryoablation therapy and immediately withdrew the probe to prevent complications associated with cryoablation. After maze operation, the patient was weaned from CPB with an infusion of dopamine and dobutamine. Protamine 310 mg was infused for 10 minutes to reverse heparin effect. The resultant ACT was 167 seconds and additional 50 mg of protamine was injected. Two units of fresh frozen plasma, eight units of platelet and remaining pump blood were transfused. The follow-up TEE examination revealed a well-functioning prosthetic tissue valve with moderate left ventricular dysfunction (EF =40%). The TEE probe was removed after the chest was closed and there was no evidence of bleeding or other trauma associated with the TEE placement. The patient was transferred to the intensive care unit (ICU) with no complication.
In the ICU, he exhibited 33–60 mmHg of mean blood pressure with 110 bpm of tachycardia, and poor urine output in spite of sufficient volume replacement with inotropic agent. Drained blood checked through the chest tube was only 10 to 40 ml/hr. Hypovolemia was suspected and eight units of red blood cells and three units of fresh frozen plasma were administered. The vital signs suggestive of hypovolemia persisted during the night, but the causative factors were not found.
On the early morning after surgery, he presented bloody secretion through the oral suction and then Levin tube was inserted into the patient. Profuse fresh blood exceeding 500 ml was drained through the Levin tube, thus endoscopy was carried out by gastroenterologists. Linear and sharp ulceration accompanied by bleeding from the gastroesophageal junction to the upper body of stomach was found on endoscopic examination (Figure 1A). Epinephrine injection did not stop the bleeding; thus, hemostasis was achieved by four clips under the endoscopy. We confirmed no bleeding spots and ended the procedure.
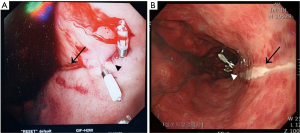
On the fifth postoperative day, he was followed up the progress with endoscopy (Figure 1B). There was a linear ulcer as before and the stomach showed erythematous change on the whole mucous. There was no active bleeding but rather a healing process. The patient was transferred to general ward and discharged on the 15th postoperative day without any other complications. The patient’s progress is shown in Figure 2 as a timeline.
Discussion
This case presents emergent gastroesophageal injury from cryoenergy with TEE manipulation. There have been many reports about either cryoablation associated gastroesophageal injury or TEE associated gastroesophageal injury.
According to the 2012 expert consensus statement, catheter ablation is a class I level A indication for the treatment of drug refractory paroxysmal atrial fibrillation and pulmonary vein isolation is the standard approach (1). The two frequently used ablation technologies for pulmonary-vein isolation are radiofrequency and cryoablation.
Cryoablation refers to all methods of destroying tissue by freezing; the aim of surgical cryoablation therapy is to create transmural lesions to eliminate focal triggers or macro reentry circuits in atrial fibrillation. The mechanism by which cryothermal energy affects the conduction tissue is well-understood. The application of a cryoprobe forms a well-demarcated hemispherical block of frozen tissue, or ice ball, which can have either oblate or prolate geometry. Cells within the iceball become irreversibly damaged and ultimately are replaced by fibrotic tissue (2-4).
Recently, Kuck et al. (5) reported that cryoablation was noninferior to radiofrequency ablation with respect to efficacy and safety. Other investigator demonstrated that cryoablation caused fewer complications of perforation or thromboembolic events compared with radiofrequency ablation and cryoablation preserves the architecture of the underlying tissue and minimizes endocardial disruption (6). However, ablation at these sites carries a risk of collateral injury to adjacent structures including the esophagus. The inferior pulmonary venous lesion is located posterior, near the esophagus, so esophageal complications associated with cryothermal energy are more likely to occur at these sites than other regions. Cryoablation itself can cause significant luminal esophageal temperature (LET) decrease, resulting in reversible esophageal ulcerations. Ahmed et al. described an esophageal effect of cryoenergy during cryoablation for atrial ablation. They monitored LET in cryoballoon catheter ablation patients and performed post-procedural endoscopy at 1±2 postoperative day. There were esophageal ulcerations in 17% of patients after cryoablation; the manner of esophageal injury in that study was ulceration, inflammation, and thickening (4). Although the risk of esophageal complications associated with cryoablation was higher, the patients in that study were asymptomatic and they did not have any sequelae. Esophageal contour changes during cryoablation of atrial fibrillation using barium enema may result from ice ball formation causing passive tissue displacement or esophageal spasm (7).
Esophageal injury often occurs with a delay and occurs most frequently on the fourth day after cryoablation (8). The delayed occurrence of esophageal injury may be explained by the same mechanism by which the cryothermal energy affects conduction tissue. The histopathologic process, particularly hemorrhagic and inflammation phase of tissue after cryoablation takes two days to one week after cryoablation.
As another possible factor, the TEE probe may also affect gastroesophageal injury. Although TEE is a useful diagnostic tool during open heart surgery, considered relatively safe and noninvasive, the TEE probe is associated with complications such as odynophagia, dental injury, endotracheal tube malposition, and gastroesophageal injury (9).
Mechanisms associated with perioperative TEE are related to direct trauma by manipulation, and indirect processes like prolonged periods of compression with the TEE probe due to lengthy procedures, tissue hypoperfusion, or ischemia due to lack of pulsatile flow and hemodynamic alterations during surgery (10). The other mechanism is thermal injury from the probe tip of TEE (11). TEE related injuries exhibit commonly as an ulceration or perforation. The upper esophagus is injured frequently during TEE probe insertion whereas mid and distal esophagus injury occurs when the TEE probe is left in the esophagus for prolonged duration (10).
Lennon et al. (12) reported the incidence of major gastrointestinal complications associated with intraoperative TEE in adult cardiac surgery patients. They said late presentation (>24 hours) was more common than early presentation (10,11).
There are many reports of TEE use in cardiac procedures. The cases of catheter ablation are increasing in number and it is very important to reduce the complications related to trans-septal catheterization such as cardiac tamponade and systemic embolism (13). Considering most complications are nearly all caused by incorrect puncture site (13), TEE can provide more benefit even when using biplane fluoroscopy. TEE not only 3D TEE allows real-time visualization of pulmonary vein anatomy, as well as of all surrounding atrial structures (14). It permits online monitoring of balloon positioning and pulmonary vein occlusion via color Doppler, which guides the operator to perform every step of the procedure in a safe manner and might improve both safety and efficacy of the procedure.
However, our case differed completely from previous reports in that it was an acute severe injury, a sharp linear lesion, a kind of laceration. The gastroenterologist concluded that the lesion was not caused by a peptic ulcer, but by scratching by a sharp material. The Figure 1A presents acute stage of esophageal bleeding after clipping of the lesions. The figure shows sharp and linear laceration on the gastroesophageal junction which is bleeding. The patient in our case presented with acute injury of the esophagus and he exhibited massive bleeding immediately after arriving ICU. Considering that we did not encounter any resistance during insertion, the manner of a sharply lacerated lesion and its acute onset, neither TEE probe nor cryoablation were the sole cause, and the injury was not a conventional injury.
Although the exact etiology of gastroesophageal injury in this case remains unknown, we assumed that the TEE probe with ice material attached, formed by extremely low temperature during cryoablation, and might tear the esophagus and stomach by manipulation of TEE probe during withdrawal.
There was no case of esophageal injury related to TEE along with cryoablation before, and rather than that, they did cryoablation using TEE successfully without any complications (15,16). In contrast, in this case, we observed that cryoablation under TEE probe is not fully safe, and we should keep in mind that this kind of complication can occur, especially during cardiac surgery with massive anticoagulant therapy. Our case demonstrated that we should manipulate the TEE probe cautiously during cryoablation. Moreover, the timing of withdrawal of TEE probe just before thawing was critical in this case. Since cryoablation in cardiac procedures is often performed with TEE inserted, we should not overlook the occurrence of esophageal injury by both cryoablation and TEE probes.
There are limitations in our case report. We did not conduct LET monitoring. Ahmed et al. compared the incidence of esophageal ulceration in patients with LET monitoring and those without LET monitoring (4). Esophageal injury was observed more frequently (36% vs. 6%, P
In conclusion, we suggest that withdrawal of the TEE probe before the cryoablation, or not to manipulate if cryoablation has already started. For the left atrial cryoablation with arrested heart, it is better not to move the TEE probe for any clinical reason; this method might be an important prophylaxis for preventing gastroesophageal injury during cardiac surgery. Moreover, for manipulation of TEE probe during cryoablation, LET monitoring might be another option for preventing gastroesophageal injury.
Patient perspective
I heard that there was a serious stomach bleeding in the ICU and bleeding was due to scratch of ice material around TEE probe. However, I understand that TEE is a useful monitoring tool during cardiac surgery and that this may result in rare but dangerous complications. They gave me proper treatment as soon as I had complications, and I am now living well without any inconvenience. I am grateful to all medical staff.
Acknowledgements
This work was supported by clinical research grant from Pusan National University Hospital.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm 2012;9:632-696.e21. [Crossref] [PubMed]
- Erinjeri JP, Clark TW. Cryoablation: mechanism of action and devices. J Vasc Interv Radiol 2010;21:S187-91. [Crossref] [PubMed]
- Lustgarten DL, Keane D, Ruskin J. Cryothermal ablation: mechanism of tissue injury and current experience in the treatment of tachyarrhythmias. Prog Cardiovasc Dis 1999;41:481-98. [Crossref] [PubMed]
- Ahmed H, Neuzil P, d'Avila A, et al. The esophageal effects of cryoenergy during cryoablation for atrial fibrillation. Heart Rhythm 2009;6:962-9. [Crossref] [PubMed]
- Kuck KH, Brugada J, Fürnkranz A, et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N Engl J Med 2016;374:2235-45. [Crossref] [PubMed]
- Bastani H, Insulander P, Schwieler J, et al. Safety and efficacy of cryoablation of atrial tachycardia with high risk of ablation-related injuries. Europace 2009;11:625-9. [Crossref] [PubMed]
- Herweg B, Ali R, Khan N, et al. Esophageal contour changes during cryoablation of atrial fibrillation. Pacing Clin Electrophysiol 2009;32:711-6. [Crossref] [PubMed]
- Ripley KL, Gage AA, Olsen DB, et al. Time course of esophageal lesions after catheter ablation with cryothermal and radiofrequency ablation: implication for atrio-esophageal fistula formation after catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2007;18:642-6. [Crossref] [PubMed]
- Kallmeyer IJ, Collard CD, Fox JA, et al. The safety of intraoperative transesophageal echocardiography: a case series of 7200 cardiac surgical patients. Anesth Analg 2001;92:1126-30. [Crossref] [PubMed]
- Arora S, Chakravarthy M, Srinivasan V. Delayed manifestation of a mid-esophageal tear with profuse hemorrhage after transesophageal echocardiogram. Echocardiography 2008;25:328-30. [Crossref] [PubMed]
- MacGregor DA, Zvara DA, Treadway RM Jr, et al. Late presentation of esophageal injury after transesophageal echocardiography. Anesth Analg 2004;99:41-4. [Crossref] [PubMed]
- Lennon MJ, Gibbs NM, Weightman WM, et al. Transesophageal echocardiography-related gastrointestinal complications in cardiac surgical patients. J Cardiothorac Vasc Anesth 2005;19:141-5. [Crossref] [PubMed]
- Roelke M, Smith AJ, Palacios IF. The technique and safety of transseptal left heart catheterization: the Massachusetts General Hospital experience with 1,279 procedures. Cathet Cardiovasc Diagn 1994;32:332-9. [Crossref] [PubMed]
- Ottaviano L, Chierchia GB, Bregasi A, et al. Cryoballoon ablation for atrial fibrillation guided by real-time three-dimensional transoesophageal echocardiography: a feasibility study. Europace 2013;15:944-50. [Crossref] [PubMed]
- Clark J, Bockoven JR, Lane J, et al. Use of three-dimensional catheter guidance and trans-esophageal echocardiography to eliminate fluoroscopy in catheter ablation of left-sided accessory pathways. Pacing Clin Electrophysiol 2008;31:283-9. [Crossref] [PubMed]
- Siklódy CH, Minners J, Allgeier M, et al. Cryoballoon pulmonary vein isolation guided by transesophageal echocardiography: novel aspects on an emerging ablation technique. J Cardiovasc Electrophysiol 2009;20:1197-202. [Crossref] [PubMed]