Video-assisted thoracoscopic lobectomy for non-small cell lung cancer in patients with severe chronic obstructive pulmonary disease
Introduction
A disease of serious harm to human health and life, lung cancer has shown evidently increasing morbidity and mortality worldwide in recent years, and ranked first in both figures in developed and developing countries (1). Although surgery has been recognized as the most effective method of treatment for early-stage non-small cell lung cancer (NSCLC), most patients with lung dysfunction, due to chronic obstructive pulmonary disease (COPD) after a history of smoking, are at a higher risk of complications after lung surgery. Therefore, a history of lung cancer with severe COPD is a contraindication to lobectomy. With the ongoing application of lung volume reduction surgery both at home and abroad, it has been shown that, after the removal of part of the lesions in lung tissue, lung function can be improved to varying extents for some patients with severe emphysema (2). An increasing number of studies have confirmed improvement in the lung function of patients with lung cancer and severe COPD following lobectomy (3-5). Those findings have shed new light on the indications for lobectomy in patients with lung cancer and COPD.
Video-assisted thoracoscopic lobectomy was first applied for the treatment of lung cancer in 1992. Its greatest advantage included the minimal invasiveness, reduced postoperative pain and less damage to the respiratory muscle and pulmonary function (6). The video-assisted thoracic surgery (VATS) has been reported (7) to allow significantly faster recovery of pulmonary functionality for in the early stages after lobectomy, compared with open-chest surgery, which further suggests that VATS protects lung function more efficiently as it causes less damage to respiratory muscles. With the wide application of VATS and continuous advancement in the technology of anesthesia, intensive care and preoperative respiratory function management, the indications for pulmonary resection are also expanding to include more and more elderly patients or long-term smokers whose lung function is already impaired. At present, favorable short- and long-term outcomes have been reported in a few studies using VATS lung resection to treat patients with lung cancer and severe COPD (8). So far, however, only a small number of such cases undergoing VATS lobectomy have been reported, and the findings are not sufficient to provide a comprehensive evaluation of the safety and effectiveness of this approach in this regard. Hence, this study is conducted to assess the safety and effectiveness of VATS lobectomy based on the findings of 61 patients with lung cancer and severe COPD who underwent this treatment in our department.
Materials and methods
Clinical data
The clinical data of patients undergoing VATS lobectomy in First Affiliated Hospital of Guangzhou Medical College from January 2000 to January 2011 were retrospectively analyzed. Sixty-one patients complicated with COPD were identified and enrolled in this study based on the GOLD classification standard for COPD (9). Upon enrollment, all participants were engaged in a series of preparation before surgery, including quitting smoking, respiratory function exercise, administration of phlegm drugs and chest physiotherapy.
Preoperative examination and surgical methods
Before surgery, all participants received physical examination, routine blood tests, ECG, cardiac color Doppler ultrasound and lower extremity deep venous color Doppler ultrasound. Respiratory function tests include pulmonary ventilation-dispersion function tests and ventilation-perfusion radionuclide scans. Coronary CT or treadmill activity tests were performed in patients with suspected coronary heart disease over the age of 60, as well as coronary interventional examination, if necessary.
Preoperative tumor staging was based mainly on the chest X-ray examination, chest CT, head and abdominal MRI, whole body bone scan, and bronchoscopy. PET/CT scans were recommended for patients considered to be stage II or above. All participants underwent VATS lobectomy and hilar and mediastinal lymph node dissection, of which the specific surgical techniques were already reported in our previous study (10).
Data collection and follow-up
The demographic data, smoking status, lung function test results, operative time, blood loss, postoperative hospital stay, postoperative chest tube residence time, postoperative tumor stage, postoperative complications, and pre- and post-operative ECOG performance status of all enrolled patients were collected. The following postoperative complications were recorded: perioperative mortality (in-hospital mortality or death of any cause in 30 days after surgery), severe complications (surgery-related: second thoracotomy due to postoperative bleeding; Respiratory: ARDS and bronchopleural fistula, pneumonia, pulmonary embolism, empyema, pulmonary edema, tracheostomy or second endotracheal intubation; Cardiac: myocardial infarction, myocardial ischemia or angina pectoris, cerebrovascular event, deep vein thrombosis; Others: acute renal failure, acute gastrointestinal bleeding, etc); and mild complications (atelectasis, postoperative air leakage for more than seven days, pleural effusion, atrial fibrillation or other arrhythmias, wound infection, etc). Long-term follow-up was conducted to identify the breathing status, tumor recurrence and survival of all patients, for a period of 1-60 months.
Statistical analysis
Measurement data were expressed as mean ± standard deviation (x±s). The chi-square test was used in the correlation analysis of changes in the ECOG performance status of the participants, and Kaplan-Meier survival analysis was conducted to identify the correlation with postoperative survival. The Cox regression model test was performed for each variable with a P value of ≤0.20 in the univariate analysis. The statistical analysis was completed in SPSS 13, with P<0.05 indicating a statistically significant difference.
Results
Clinical data
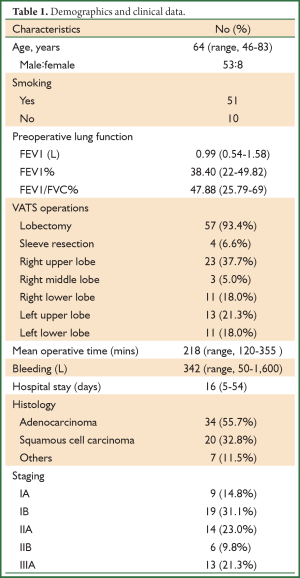
Sixty-one cases were finally included in the retrospective study, including 53 men (86.9%) and eight women (Table 1). The average age was 64 years (46-83 years). Fifty-one patients were long-term smokers. The preoperative FEV1/FVC was <70% and FEV1% <50% in all patients, with a mean preoperative FEV1 of 0.99 L (0.54-1.58 L) and mean FEV1% of 38.4% (22-49.82%).
Full Table
All of the 61 patients underwent the VATS lobectomy or sleeve resection plus systemic lymph node dissection [right upper lobe in 23 cases (37.7%), right middle lobe in three (5.0%), right lower lobe in eleven (18.0%), left upper lob in thirteen (21.3%) and left lower lobe in eleven (18.0%)]. The mean operative time was 218 minutes (120-355 minutes), with a mean intraoperative blood loss of 342 mL (50-1,600 mL). None of the patients converted to thoracotomy. Postoperative pathology reported 34 cases of adenocarcinoma (55.7%), 20 cases of squamous cell carcinoma (32.8%) and seven of other tumors (11.5%). All participants were subject to pathological and clinical staging according to the TNM Classification of the UICC, 7th edition (11). As a result, there were nine patients of IA (14.8%), nineteen of IB (31.1%), fourteen of IIA (23.0%), six of IIB (9.8%), and thirteen of IIIA (21.3%).
Complications after surgery
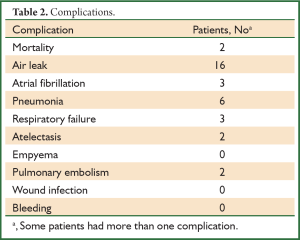
Two patients died of ARDS during the perioperative period, and 24 patients (39.3%) presented postoperative complications (Table 2). Twenty-two patients (36.1%) had respiratory complications postoperatively, including air leakage in 16 cases (25.8%), pulmonary infection in six, respiratory failure in three, atelectasis in two and pulmonary embolism in two. The average hospital stay was 16±1.1 days (5-54 days).
Full Table
Overall survival
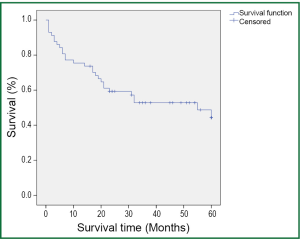
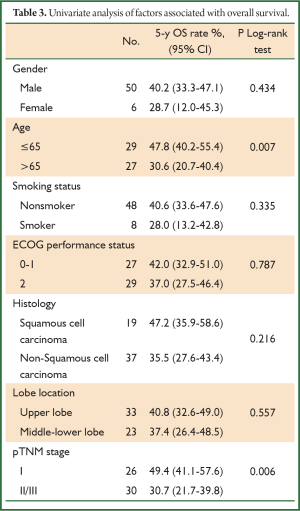
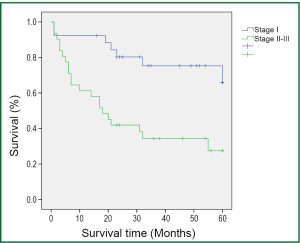
During a median follow-up time of 39 months (1-60 months), five patients were lost to follow-up and 27 died. The survival rate was 75.4% in the first year, and 50.9% in five years (Figure 1). In the univariate analysis using the Log-rank test, the outcomes were correlated with age and postoperative TNM staging (P=0.007 and 0.006, Table 3). The median survival of patients not older than 65 years was 48 months, and reduced to 31 months in those older than 65 (P=0.007, Figure 2). Patients with stage I tumors had a median survival of 49 months, while those had stage II/III tumors had only 28 months. The difference was significant between them (P=0.006, Figure 3). In the Cox regression model, when taking into account those factors showing significant effect on survival in the univariate analysis, age and TNM staging after tumor resection were independent predictive factors for the 5-year survival in those patients (P=0.014 and 0.013, Table 4).
Full Table

Full Table
The ECOG scores were recorded three months before and after surgery to evaluate the changes of lung function and quality of life for the patients (12). The results showed that mean ECOG scores of 1.51 and 1.31 before and after surgery, respectively, among the 59 patients, excluding two who died during the perioperative period. The difference between those scores was significant (P<0.05).
Discussion
Lung cancer and COPD are two common diseases of human beings. The presence of both conditions in a patient can increase the risk of complications after lung surgery due to underlying lung function damage. Since lung cancer patients with severe COPD are at a higher risk of postoperative complications, most of they have to receive non-radical partial lung resection (wedge or segmental resection) instead of lobectomy, which is currently recognized as the most effective means of treatment for early stage lung cancer. For patients with lung cancer, however, both pulmonary wedge resection and segmental resection are associated with a significantly increased recurrence rate and lower postoperative survival compared with standard lobectomy (13,14).
With the ongoing application of lung volume reduction surgery, it has been found that partial lung resection can achieve the similar result to volume reduction for patients with lung cancer and emphysema (3), which can minimize or even improve postoperative pulmonary function loss. Those findings have shed new light on the surgical options for patients with lung cancer and severe COPD. With the development of surgical techniques, anesthesia and intensive medical technology, an increasing number of studies have reported that lung resection can be tolerated by patients with lung cancer and severe pulmonary insufficiency, and can lead to satisfying outcomes (3,8,15-18).
Since the early 1990s, VATS has been rapidly developed and widely applied in the world, involving almost all areas of general thoracic surgery. Compared with thoracotomy, VATS enables a smaller incision without removing or stretching the ribs open, sparing respiratory muscles from injures and thus minimizing the loss of lung function. Moreover, with a smaller incision, patients will suffer less pain postoperatively and expectorate more easily, reducing the incidence of postoperative pulmonary infection and complications as well. In view of those advantages, VATS procedures have been used in a growing number of studies to treat patients with lung cancer and severe pulmonary dysfunction (8,19).
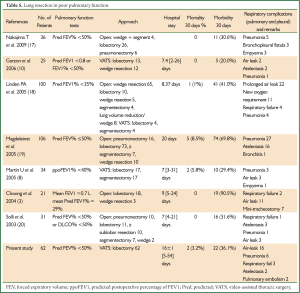
Previous studies have shown that, however, patients with lung cancer and COPD have an increased risk of cardiopulmonary complications compared to patients with lung cancer alone (20). In the present study, two patients died of respiratory failure in the perioperative period and 24 patients (39.3%) had postoperative complications, of which 22 (36.1%) had respiratory complications with an average hospital stay of 16 days after surgery. It can be seen that the incidence of postoperative respiratory complications in this study is not unacceptable compared with the previous reports (Table 5). According to the existing studies, open chest surgery is associated with a longer postoperative hospital stay and higher incidence of respiratory complications in patients with lung cancer and severe pulmonary dysfunction compared with the VATS procedures, which further demonstrates that the VATS technique is an ideal option for such patients. A possible explanation for the lower risk of postoperative pulmonary complications is that reduced injury to respiratory muscles, smaller chest wall incision and consequently less pain allows patients to cough and expectorate more easily and get out of bed sooner after VATS, and this in turn reduces the likelihood of other complications of the respiratory system. In the present study, pulmonary complications were observed in 36.1% of the patients, which is lower than the report of most studies with open chest surgery (3,16,17) but higher than those with VATS surgery (8). Martin et al. (8) carried out VATS lung resection for 34 patients with lung cancer and a FEV1% <40%; Although there were two dead cases, respiratory complications were observed in only ten patients (29.4%). In the present study, although the incidence of postoperative respiratory complications was higher than the above findings (8), systemic radical surgery was administered to all of the patients with lung cancer and severe COPD in the former, while VATS lobectomy accounted for up to 50.2% and 50% (8) in the other two studies. Lobectomy is associated with much greater surgical injury and loss of functional alveolar areas than either wedge resection or segmentectomy, and there were seven patients with extremely severe COPD and a preoperative FEV1% of only 27.8% (22-29.9%) in this study.
Full Table
Patients in this study had a relatively long hospital stay, averaging 16 days. Although it is slightly shorter than 20 days as reported by Magdeleinat et al. (17), it is longer than all of the other studies, which may be largely due to the surgical approaches. In this study, all 61 patients received either lobectomy or sleeve resection, whereas lobectomy accounts for a relatively small part in all of the remaining studies.
In the present study, both short- and long-term survival rates are observed in patients with moderate COPD who received lobectomy or sleeve resection after a 5-year follow-up. The survival analysis showed a 1-year survival rate of 75.4%, which was basically consistent with the findings of Magdeleinat (17), and a 5-year survival rate of 50.9%, which was higher than the report of Magdeleinat. Further analysis showed significantly better outcomes in patients with stage I lung cancer than in those with stages II or III, with the 5-year survival rates being 73.1% and 32.3%, respectively (P<0.05), which were generally consistent with other reports (8,15,17). According to the report by Martin et al. (8), the analysis of 34 patients with stage I lung cancer and severe pulmonary dysfunction who underwent VATS lobectomy or segmental resection revealed a 5-year survival up to 69.7%, without significant difference between the two groups. Nakajima et al. (15) found a 5-year survival of 57.9% in the stage I group as a part of 36 patients with lung cancer and severe lung dysfunction, but the 5-year survival was merely 11.9% in the more advanced groups.
Lung cancer and COPD are mostly found in elderly people, while patients over the age of 65 years account for about 50% and those over the age of 70 years account for 30-40% of all cases (21). COPD and cardiovascular diseases are the common concomitant diseases in elderly smokers with lung cancer, and the presence of such conditions may directly or indirectly affect their therapy and outcomes. In the study of Janssen-Heijnen et al. (22), age was regarded as an independent factor for the survival outcomes of patients with stages I and II NSCLC, though it had no significant impact on the survival outcomes of patients at more advanced stages. Li et al. (23) also found that the 5-year survival rate was significantly higher in patients with stage I lung cancer who were not older than 65 years, compared with those older. In our previous study, we also found that age could be a critical factor in predicting the outcomes of those patients (24). A number of studies (15,17,25) have shown that, for patients complicated with severe pulmonary dysfunction, those with stage I lung cancer would have a better outcome than patients with the condition at stages II and III (P<0.05). In the present study, multivariate statistical analysis also suggested that age could be an independent prognostic factor for patients with lung cancer and severe COPD, which was consistent with previous reports.
However, there are several limitations in this study due to its retrospective nature. Although it has included the largest number of patients with lung cancer and severe COPD undergoing VATS lobectomy so far, the absolute number is not significantly large. Secondly, the present analysis included only the 5-year survival but not the time to progression, and did not take into account the subsequent treatment patients received after the surgery when calculating the 5-year survival rates. Finally, an objective comparison between the lung function data before and after the surgery is unavailable because some of patients did not receive postoperative pulmonary function tests. Hence, the changes in the quality of life can merely be analyzed based on some relatively objective indicators in the present study. A more comprehensive prospective study will be needed to further determine the safety and effectiveness of VATS lobectomy as the treatment for patients with lung cancer and severe COPD.
In conclusion, VATS lobectomy can be safely and effectively performed for patients with NSCLC and severe COPD to achieve a satisfying long-term survival outcome as good as the routine VATS procedure, with an acceptable incidence of postoperative complications. Therefore, our preliminary conclusion is that for younger patients at an earlier stage (stage I), VATS lobectomy can be used as a more effective treatment option.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Zeng Q, Jiang S. Update in diagnosis and therapy of coexistent chronic obstructive pulmonary disease and chronic heart failure. J Thorac Dis 2012;4:310-5. [PubMed]
- Choong CK, Meyers BF, Battafarano RJ, et al. Lung cancer resection combined with lung volume reduction in patients with severe emphysema. J Thorac Cardiovasc Surg 2004;127:1323-31. [PubMed]
- Zhong N. Nipping it in the bud: An inspiring mission for prevention and management of COPD. J Thorac Dis 2012;4:102-5. [PubMed]
- Korst RJ, Ginsberg RJ, Ailawadi M, et al. Lobectomy improves ventilatory function in selected patients with severe COPD. Ann Thorac Surg 1998;66:898-902. [PubMed]
- Richards JMJ, Dunning J, Oparka J, et al. Video-assisted thoracoscopic lobectomy: the Edinburgh posterior approach. Ann Cardiothorac Surg 2012;1:61-9. [PubMed]
- Hennon MW, Demmy TL. Video-assisted thoracoscopic surgery (VATS) for locally advanced lung cancer. Ann Cardiothorac Surg 2012;1:37-42. [PubMed]
- Martin-Ucar AE, Nakas A, Pilling JE, et al. A case-matched study of anatomical segmentectomy versus lobectomy for stage I lung cancer in high-risk patients. Eur J Cardiothorac Surg 2005;27:675-9. [PubMed]
- Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001;163:1256-76. [PubMed]
- He J, Shao W, Cao C, et al. Long-term outcome and cost-effectiveness of complete versus assisted video-assisted thoracic surgery for non-small cell lung cancer. J Surg Oncol 2011;104:162-8. [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [PubMed]
- Berrisford R, Brunelli A, Rocco G, et al. The European Thoracic Surgery Database project: modelling the risk of in-hospital death following lung resection. Eur J Cardiothorac Surg 2005;28:306-11. [PubMed]
- Warren WH, Faber LP. Segmentectomy versus lobectomy in patients with stage I pulmonary carcinoma. Five-year survival and patterns of intrathoracic recurrence. J Thorac Cardiovasc Surg 1994;107:1087-93; discussion 1093-4. [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [PubMed]
- Nakajima T, Sekine Y, Yamada Y, et al. Long-term surgical outcome in patients with lung cancer and coexisting severe COPD. Thorac Cardiovasc Surg 2009;57:339-42. [PubMed]
- Linden PA, Bueno R, Colson YL, et al. Lung resection in patients with preoperative FEV1 <35% predicted. Chest 2005;127:1984-90. [PubMed]
- Magdeleinat P, Seguin A, Alifano M, et al. Early and long-term results of lung resection for non-small-cell lung cancer in patients with severe ventilatory impairment. Eur J Cardiothorac Surg 2005;27:1099-105. [PubMed]
- Solli P, Leo F, Veronesi G, et al. Impact of limited pulmonary function on the management of resectable lung cancer. Lung Cancer 2003;41:71-9. [PubMed]
- Demmy TL, Curtis JJ. Minimally invasive lobectomy directed toward frail and high-risk patients: a case-control study. Ann Thorac Surg 1999;68:194-200. [PubMed]
- Pompili C, Brunelli A, Refai M, et al. Does chronic obstructive pulmonary disease affect postoperative quality of life in patients undergoing lobectomy for lung cancer? A case-matched study. Eur J Cardiothorac Surg 2010;37:525-30. [PubMed]
- Yancik R. Cancer burden in the aged: an epidemiologic and demographic overview. Cancer 1997;80:1273-83. [PubMed]
- Janssen-Heijnen ML, Smulders S, Lemmens VE, et al. Effect of comorbidity on the treatment and prognosis of elderly patients with non-small cell lung cancer. Thorax 2004;59:602-7. [PubMed]
- Li Z, Yu Y, Lu J, et al. Analysis of the T descriptors and other prognosis factors in pathologic stage I non-small cell lung cancer in China. J Thorac Oncol 2009;4:702-9. [PubMed]
- Wang W, Xu X, Wang W, et al. The expression and clinical significance of CLIC1 and HSP27 in lung adenocarcinoma. Tumour Biol 2011;32:1199-208. [PubMed]
- Lau KK, Martin-Ucar AE, Nakas A, et al. Lung cancer surgery in the breathless patient--the benefits of avoiding the gold standard. Eur J Cardiothorac Surg 2010;38:6-13. [PubMed]