Vasopressor hormones in shock—noradrenaline, vasopressin or angiotensin II: which one will make the race?
Currently, adrenergic substances—catecholamines—are the vasoconstrictor agents most frequently used in critically ill patients suffering from vasodilatory hypotension and tissue hypoperfusion (1). Their therapeutic safety margin is small (2). Dose dependently, adverse cardiac events occur in up to 50% of critically ill patients exposed to catecholamine therapy and are associated with an increase in both morbidity and mortality (3). Aside from unspecific nitric oxide synthase inhibitors [e.g., methylene blue (4)], vasopressin derivatives such as arginine vasopressin or glycylpressin have been clinically evaluated as alternative vasopressor agents to adrenergic agents (5-7). The multicentred VASST trial did not find a mortality difference between septic shock patients treated with a combination of arginine vasopressin and norepinephrine compared with norepinephrine alone but suggested there might be a survival benefit when vasopressin was added to norepinephrine before dose requirements exceeded 15 µg/min (8). Both arginine vasopressin and glycylpressin have been associated with serious side effects (9,10) and are more difficult to titrate than norepinephrine or epinephrine. This implies that the quest for the optimal vasopressor agent in vasodilatory shock is still ongoing.
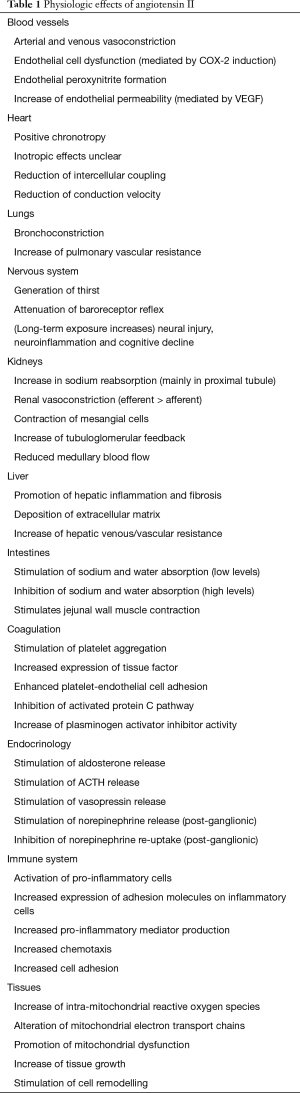
Angiotensin II is the main vasoconstrictor hormone of the renin-angiotensin-aldosterone system. It has extensively been examined in its role as a pathophysiologic factor in essential arterial hypertension and (post myocardial infarction) chronic heart failure (11,12). Physiologic effects of angiotensin II are largely mediated through G-protein coupled AT1 receptors although other receptors are involved as well (e.g., AT2, AT4, Mas) (Table 1). Except for small clinical studies and one clinical trial (13-15), the use of angiotensin II as a vasopressor agent in patients with shock has so far not been systematically addressed. The newly published Angiotensin II for the Treatment of Vasodilatory Shock (ATHOS-3) trial evaluated the effectiveness of angiotensin II as a vasopressor drug in vasodilatory shock (16).
Full table
The trial was conducted as a prospective, randomized, double-blinded study at 75 intensive care units in nine countries from May 2015 until January 2017 (NCT02338843). Critically ill patients aged >18 years and suffering from vasodilatory shock requiring norepinephrine (or equivalents) at doses >0.2 µg/kg/min were eligible for study inclusion. Vasodilatory shock was defined as arterial hypotension [mean arterial pressure (MAP) 55–70 mmHg] and a cardiac index ≥2.3 L/min/m2 or a combination of a central venous oxygen saturation >70% and a central venous pressure >8 mmHg. During the preceding 24 h, at least 25 mL/kg of fluids must have been infused. Following enrollment, patients were randomized to receive a continuous intravenous infusion of either synthetic angiotensin II (LJPC-501) or normal saline. During the first three h, blinded study drugs were titrated to achieve a MAP ≥75 mmHg. The doses of previously installed vasopressors were held constant during this study phase. Over the subsequent 45 h, the doses of the study drug and open vasopressor agents were adjusted to maintain MAP between 65 and 75 mmHg. The primary endpoint of the study was the percentage of patients in whom, during the first 3 h after randomization, MAP could be increased ≥75 mmHg or at least by 10 mmHg compared to pre-randomization values. Based on the (hypothetical) assumption that the primary endpoint could be achieved in 60% in the angiotensin II and in 40% in the placebo group, 150 subjects were projected to be included in each study group to detect a significant between group difference with more than 90% power and at a two-sided alpha-level of 0.05. The ATHOS-3 trial was sponsored by the La Jolla Pharmaceutical company and is part of the FDA registration of LJPC-501. The sponsor was involved in the design of the study protocol, performed the statistical analysis, and paid for a professional medical writer to assist with the revision of the manuscript.
Of 404 subjects screened, 344 patients were enrolled into the trial. Twenty-three of these patients were randomized but never received a study drug, mostly because of a rapid improvement of their hemodynamic function. Hundred-sixty-three patients in the angiotensin II and 158 patients in the placebo group were eventually included into the statistical analysis using a modified intention-to-treat approach. The primary study endpoint was accomplished in 69.9% of patients randomized to the angiotensin II group and 23.4% allocated to the placebo group (OR =7.95; 95% CI, 4.76–13.3; P<0.001). In the angiotensin II group, MAP could be elevated ≥75 mmHg more frequently in patients with norepinephrine requirements <0.5 µg/kg/min compared to those requiring higher doses (77.8% vs. 50%; P<0.001). The relative increase in MAP during the first three h of the intervention was higher in study than control patients (12.5 vs. 2.9 mmHg; P<0.001). Previously installed vasopressor agents could be reduced more frequently and extensively in study than control patients. Except for heart rate, which significantly increased during angiotensin II infusion, the course of no other hemodynamic parameter of the study patients was presented. The secondary study endpoint (mean change in the cardiovascular SOFA score) was achieved more frequently in patients treated with angiotensin II than those receiving placebo. Changes in the total SOFA score over the 48 h observation period did not differ between groups. The rate of adverse events (study group, 87.1%; control group, 91.8%) and 28-day mortality (study group, 46%; control group, 54%; HR =0.78; 95% CI, 0.57–1.07; P=0.12) were comparable between the study and control group. The authors concluded that angiotensin II effectively increased arterial blood pressure in patients with vasodilatory shock that did not respond to high doses of conventional vasopressors.
The conclusions are well supported by the results of the trial. The study design is appropriate to test the effectiveness of angiotensin II to increase arterial blood pressure in vasodilatory shock. The number of patients included was high and clearly sufficient to answer the primary study question. Although the rate of adverse events did not differ between groups, the hypothetical assumption that normal saline would relevantly increase MAP in 40% of patients with advanced vasodilatory shock appears interestingly high and retrospectively exposed a large number of patients to a drug with a previously unknown risk profile (17). The main point of critique of the ATHOS-3 trial is the definition of vasodilatory shock. While the clinically accepted definition of vasodilatory shock of arterial hypotension despite a normal or increased cardiac index was fulfilled by only 44.2% of study patients (namely by those in whom cardiac index was measured), the combination of arterial hypotension with a central venous oxygen saturation >70% and a central venous pressure >8 mmHg was used to assume that more than half of the study patients suffered from vasodilatory shock. Both central venous oxygen saturation and central venous pressure are neither appropriate nor validated to diagnose or define vasodilatory shock. It is, therefore, possible that vasodilatory hypotension was not the leading hemodynamic pathology in a substantial number of patients enrolled in the trial. Experimental studies have shown that angiotensin II exerts relevantly different hemodynamic effects in healthy and pathologic conditions (18,19). During the first three h after randomization, the dose of angiotensin II peaked at a mean dose of 37 ng/kg/min. Even though the study protocol allowed increases of the angiotensin II dose up to 200 ng/kg/min, an increase in MAP ≥75 mmHg during this period could only be achieved in half of the patients who required very high doses of norepinephrine (>0.5 µg/kg/min). Comparisons of the hemodynamic effects of angiotensin II with vasopressin derivatives are not possible based on these data. Previous studies, however, reported consistent increases of both systemic vascular resistance and arterial blood pressure during arginine vasopressin infusion in patients with advanced vasodilatory shock and norepinephrine requirements >0.5 µg/kg/min (6).
Although the primary study endpoint of the trial focused on the arterial blood pressure response to angiotensin II infusion, MAP does not correlate with tissue perfusion and microcirculatory blood flow in patients with severe sepsis or septic shock (20). When assessing the effectiveness of a vasopressor agent, it is essential to understand the mechanisms through which it increases arterial blood pressure (e.g., through increases in systemic vascular resistance, cardiac output or both). Unfortunately, the present publication of the ATHOS-3 trial group does not reveal data on the course of cardiac index or systemic vascular resistance during angiotensin II treatment. The only hemodynamic parameter whose course during the study intervention was reported is the heart rate. Absolute heart rates were higher in patients treated with angiotensin II compared with placebo. The increase in heart rate was particularly pronounced during the first 6 h after randomization and can physiologically be explained by positive chronotropic effects of angiotensin II mediated by cardiac AT2-receptor stimulation. The overall rate of tachyarrhythmias in the study population was very low and did not differ between patients in the angiotensin II and control group (9.2% vs. 7.6%; OR =1.23; 95% CI, 0.52–2.92; P=0.27). Tachycardic effects related to the use of angiotensin II appear particularly problematic in light of the fact that tachycardia and tachyarrhythmias are common in and serious complications of patients with shock (21,22).
Since the study intervention was terminated after 48 h, the ATHOS-3 trial results do not allow conclusions regarding the effects of angiotensin II on organ function, the length of stay in the intensive care unit or patient survival. These essential outcome parameters need to be appraised by future prospective trials evaluating the use of angiotensin II in patients with vasodilatory shock. As the pathophysiology of vasodilatory shock delicately differs between patients with or without sepsis (e.g., extent of downregulation of vasoconstrictor receptors, degree of microcirculatory dysfunction), it is important that these future trials focus on patient groups suffering from one specific type of vasodilatory shock such as either septic or postcardiotomy vasodilatory shock.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torgersen C, Dünser MW, Schmittinger CA, et al. Current approach to the haemodynamic management of septic shock patients in European intensive care units: a cross-sectional, self-reported questionnaire-based survey. Eur J Anaesthesiol 2011;28:284-90. [PubMed]
- Dünser MW, Hasibeder WR. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med 2009;24:293-316. [Crossref] [PubMed]
- Schmittinger CA, Torgersen C, Luckner G, et al. Adverse cardiac events during catecholamine vasopressor therapy: a prospective observational study. Intensive Care Med 2012;38:950-8. [Crossref] [PubMed]
- Belletti A, Musu M, Silvetti S, et al. Non-Adrenergic Vasopressors in Patients with or at Risk for Vasodilatory Shock. A Systematic Review and Meta-Analysis of Randomized Trials. PLoS One 2015;10:e0142605. [Crossref] [PubMed]
- Landry DW, Levin HR, Gallant EM, et al. Vasopressin pressor hypersensitivity in vasodilatory septic shock. Crit Care Med 1997;25:1279-82. [Crossref] [PubMed]
- Dünser MW, Mayr AJ, Ulmer H, et al. The effects of vasopressin on systemic hemodynamics in catecholamine-resistant septic and postcardiotomy shock: a retrospective analysis. Anesth Analg 2001;93:7-13. [Crossref] [PubMed]
- O'Brien A, Clapp L, Singer M. Terlipressin for norepinephrine-resistant septic shock. Lancet 2002;359:1209-10. [Crossref] [PubMed]
- Russell JA, Walley KR, Singer J, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 2008;358:877-87. [Crossref] [PubMed]
- Malay MB, Ashton JL, Dahl K, et al. Heterogeneity of the vasoconstrictor effect of vasopressin in septic shock. Crit Care Med 2004;32:1327-31. [Crossref] [PubMed]
- Dünser MW, Mayr AJ, Tür A, et al. Ischemic skin lesions as a complication of continuous vasopressin infusion in catecholamine-resistant vasodilatory shock: incidence and risk factors. Crit Care Med 2003;31:1394-8. [Crossref] [PubMed]
- Touyz RM. The role of angiotensin II in regulating vascular structural and functional changes in hypertension. Curr Hypertens Rep 2003;5:155-64. [Crossref] [PubMed]
- Hirsch AT, Pinto YM, Schunkert H, et al. Potential role of the tissue renin-angiotensin system in the pathophysiology of congestive heart failure. Am J Cardiol 1990;66:22D-30D; discussion 30D-32D.
- Cohn JN, Luria MH. Studies in Clinical Shock and Hypotension. Ii. Hemodynamic Effects of Norepinephrine And Angiotensin. J Clin Invest 1965;44:1494-504. [Crossref] [PubMed]
- Derrick JR, Anderson JR, Roland BJ. Adjunctive use of a biologic pressor agent, angiotensin, in management of shock. Circulation 1962;25:263-7. [Crossref] [PubMed]
- Chawla LS, Busse L, Brasha-Mitchell E, et al. Intravenous angiotensin II for the treatment of high-output shock (ATHOS trial): a pilot study. Crit Care 2014;18:534. [Crossref] [PubMed]
- Khanna A, English SW, Wang XS, et al. Angiotensin II for the Treatment of Vasodilatory Shock. N Engl J Med 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Kimmoun A, Levy B. Angiotensin II: a new approach for refractory shock management? Crit Care 2014;18:694. [Crossref] [PubMed]
- Pereira AJ, Jeger V, Fahrner R, et al. Interference of angiotensin II and enalapril with hepatic blood flow regulation. Am J Physiol Gastrointest Liver Physiol 2014;307:G655-63. [Crossref] [PubMed]
- Corrêa TD, Jeger V, Pereira AJ, et al. Angiotensin II in septic shock: effects on tissue perfusion, organ function, and mitochondrial respiration in a porcine model of fecal peritonitis. Crit Care Med 2014;42:e550-9. [Crossref] [PubMed]
- De Backer D, Creteur J, Preiser JC, et al. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med 2002;166:98-104. [Crossref] [PubMed]
- Leibovici L, Gafter-Gvili A, Paul M, et al. Relative tachycardia in patients with sepsis: an independent risk factor for mortality. QJM 2007;100:629-34. [Crossref] [PubMed]
- Walkey AJ, Wiener RS, Ghobrial JM, et al. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA 2011;306:2248-54. [Crossref] [PubMed]


