Bronchial hyperreactivity in perimenstrual asthma is associated with increased Th-2 response in lower airways
Introduction
Asthma, one of the most common chronic respiratory diseases might deteriorate during perimenstrual period, representing a phenomenon known as perimenstrual asthma (PMA) (1). PMA occurs in 8.2% to 57% of asthmatic women in childbearing age (2-4) and has often more severe course than other asthma phenotypes (5-7). However, current knowledge about the pathogenesis of PMA is incomplete and inconsistent. Most, but not all (8) data, obtained from studies conducted so far underlie the hypothesis, that links cycle-related changes of female steroid sex hormones with changing concentrations of proinflammatory mediators in both, peripheral blood and lower airways of patients with PMA (9-11).
Increased bronchial hyperreactivity has been suggested as one of the most important background factors for the exacerbation of asthma during perimenstrual period (12). Tan et al. demonstrated that airway responsiveness to adenosine 5’-monophosphate (AMP) in asthmatic women was highest during the premenstrual period (13). Previously performed studies showed, that both, estrogen and progesterone are involved in modifying airway responsiveness (14-19) which may suggest, that PMA exacerbations could be triggered by some characteristic pattern of female sex hormones concentrations during perimenstrual period. According to some studies, PMA also seems to be closely linked to some atopy markers. In study by Pereira-Vega et al. all women diagnosed with PMA had total blood immunoglobulin E (IgE) values increased above normal (20).
Data obtained in human asthma patients indicate the important role of eotaxin, IL-4 and IL-5 in mediating airway hyperresponsiveness and inflammatory cell recruitment into lower airways (21-23). However, data regarding the concentrations of inflammatory markers in lower airways of PMA patients are extremely scarce. In their study Nakasato et al. revealed that serum levels of leukotriene C4 (LTC4) [but not IL-1beta, IL-4, IL-5, IL-6, Granulocyte-macrophage colony-stimulating factor (GM-CSF), histamine, LTB4 and PAF] were significantly higher during perimenstrual exacerbations of asthma than after recovery (11). De Oliveira found less inflammatory cells in the airways of ovariectomized rats, than in the healthy controls (24). In this study, administration of estradiol was associated with the influx of inflammatory cells into the bronchoalveolar lavage and increased release of IL-1β and tumor necrosis factor (TNF) by (bronchoalveolar lavage) BAL cells (24). Similarly, progesterone administration significantly increased the release of IL-10, IL-1β, TNF by BAL cells. Oguzulgen revealed higher levels of exhaled air nitric oxide (eNO) concentration and increased induced sputum eosinophil count before the expected menstruation in PMA patients (25).
Since progesterone has been proved to directly enhance T cell differentiation into Th2 cells (26) and increased concentrations of Th2-derived cytokines (e.g., IL-4, IL-5) as well as eotaxin are believed to participate in the development of airway hyperresponsiveness, we hypothesized that increased concentration of these cytokines in lower airways of PMA patients (caused by higher progesterone concentration in luteal phase of the cycle) is responsible for asthma deterioration during this period. To test this hypothesis we measured levels of these cytokines in lower airways of PMA patients in luteal phase of menstrual cycle and compared them with non-PMA asthmatics and healthy controls.
Methods
Study population
A three-arm, case-control clinical study (women in childbearing age with PMA vs. non-PMA asthmatics vs. healthy control group) was designed. The informed, written consent was obtained from all study participants. The study was approved by ethics committee of the Medical Faculty, Silesian Medical University, Katowice, Poland.
Thirty one premenopausal women with regular menstrual cycles from the outpatient pulmonology and gynaecology units of the Central Clinical Hospital of the Silesian Medical University and from neighbouring allergy outpatient clinic were screened for PMA symptoms. Of these, 12 fulfilled entry criteria and were recruited to PMA group. PMA was diagnosed on the basis of typical clinical history of asthma [mild to severe according to current GINA recommendations (27), confirmed by the positive metacholine challenge test] with cyclical clinical asthma deterioration during luteal phase and/or during the first days of menstruation and/or ≥20% reduction of peak expiratory flow (PEF) values up to 5 days before, or during menstruation, for at least 5 consecutive years. PMA patients were all treated according to current GINA guidelines (27) and were told not to withdraw any asthma controlling drugs during the study. Other exclusion criteria for entry were: (I) age <18 and >45 year; (II) irregular menstrual cycles (cycle-to-cycle variability >3 days); (III) use of oral contraceptives in a period shorter than 6 months before recruitation; (VI) pregnancy and breast-feeding; (V) current smoking and/or history of >5 pack-years; (VI) history of infection for 4 weeks prior to the recruitation and in course of the study; (VII) any chronic diseases other than asthma; (VIII) poor patient compliance.
Each out of the nine non-PMA asthmatics recruited in the study had a typical clinical history of asthma [mild to severe according to current GINA recommendations (27), confirmed by the positive metacholine challenge test] with no exacerbations of clinical symptoms and/or PEF reduction 5 days before, or during menstruation.
Ten control subjects volunteered to take part in the study. They had no symptoms and no history of asthma or any other chronic disease and did not use any local or systemic medication.
Exclusion criteria for both, non-PMA asthmatics and healthy controls were the same as for the PMA patients.
Study design
Each of the women recruited to one of the three groups studied has prospectively been followed for 10 weeks over two consecutive menstrual cycles. At least 2 months prior to study, bronchial responsiveness (BR) to metacholine was performed in each subject. All women completed asthma symptom questionnaire, asthma control test and recorded PEF twice daily during both menstrual cycles. Total IgE serum concentrations as well, as sputum induction were determined in the 26th day of each, of the two cycles. Sputum concentration of eotaxin, IL-4 and IL-10 were measured by sandwich ELISA test. The averaged results obtained from two consecutive cycles have been subjected to statistical analysis.
Lung function and metacholine challenge test
Lung function was measured with dry spirometer (MasterLab, Jaeger, Germany) according to the recommendations of the European Respiratory Society (28).
Bronchial hyperreactivity in metacholine challenge testing was assessed according to the protocol described by Hargreave et al. (29).
Sputum induction and analysis
Sputum was induced by hypertonic saline inhalation (3%, 4% and 5% for 7 min) by an ultrasonic nebulizer device (Thomex MB, Medbryt, UK) and processed according to a method previously described (30).
Measuring cytokine levels
Sputum eotaxin, IL-4, and IL-10 concentrations were measured by ELISA kits (R & D Systems, McKinley Place N. E. Minneapolis, USA) according to the manufacturer’s protocol.
Statistics
Statistical evaluation was performed with software package (Statistica 6.0). Kolmogorov-Smirnov test was used to test variables for normal distribution. Student t test or Wilcoxon test were used to determine the differences between parameters in the different phases of the menstrual cycle. Inter-group comparisons were performed using Kruskal-Wallis ANOVA and Mann-Whitney U test. Spearman`s rank correlation test was used to evaluate correlations between different parameters. P values of <0.05 were accepted as statistically significant.
Results
Baseline characteristics, PC20 and total serum IgE
Baseline characteristics of all patients included is summarised in Table 1. Groups were comparable with respect to age, smoking status and FEV1%VC values. Asthma control measured by asthma control test (medians and ranges are in brackets) was significantly (P<0.05) worse in PMA group: 17 points (11–23 points) than in non-PMA asthmatics: 22 points (19–25 points), while the average daily use of inhaled steroids in PMA patients was significantly higher than among non-PMA asthmatics [800 vs. 400 mcg budesonide (BUD)/day, P<0.05] (31).

Full table
In metacholine challenge test PC20 values were significantly lower in both, PMA and non-PMA asthmatics, than in healthy control group (P=0.001). When compared to non-PMA asthmatics, PC20 values to metacholine in PMA group was also significantly lower (P=0.044; Table 1).
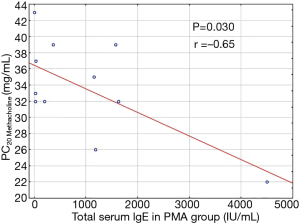
Inter-group comparisons revealed significant differences in total serum IgE level (P=0.022), with a higher total IgE concentration in PMA, than in non-PMA asthmatics (P=0.032; Table 1). In PMA group total IgE concentration in luteal phase of the menstrual cycle was reversely correlated with PC20 values (P=0.030; r =−0.65) (Figure 1).
Induced sputum cytokine levels
Measurable concentrations of eotaxin and IL-4 were found in all sputum samples assayed. Sputum IL-10 levels were undetectable in one person in PMA group and in one non-PMA asthmatic. Sputum cytokine levels in groups studied are presented in Table 2.

Full table
Sputum eotaxin and IL-4 concentrations in luteal phase of the menstrual cycle in PMA subjects were significantly higher than in non-PMA asthmatics (P=0.016; P=0.041, respectively) and healthy control group (P<0.001, both cytokines) (Table 2). No differences (P>0.05) were noticed in sputum levels of IL-10 between all groups studied (Table 2).
Discussion
PMA is one of the asthma phenotypes wherein the disease worsens cyclically in the luteal or perimenstrual phase of the cycle, leading to a recurrent loss of the disease control. Although, according to the results of epidemiological studies, PMA occurs in 8.2% to 57% of asthmatic women in childbearing age (1-4) and the disease exacerbations have often more severe course, than in other asthma phenotypes (5-7), the phenomenon of PMA still remains unclear. It is suggested that the exacerbations of asthma symptoms in PMA are associated with perimenstrual, increased airway hyperresponsiveness (12,13), dependent on fluctuating concentrations of sex hormones (9-11,14,17-19) and proinflammatory mediators (11,24,25). However, of the few studies conducted so far on the molecular basis of PMA, most focused on the markers of systemic inflammation (11,20) or was carried out in animal models (17,19,24). With this in mind, we designed a study to evaluate the possible involvement of proinflammatory cytokines in lower airways of PMA patients in the development of airway hyperresponsiveness and the co-related disease exacerbation in perimenstrual period. Additionally, in our study we compared markers of inflammation in the lower respiratory tract in patients with PMA and non-PM asthmatics, in order to better understand the molecular basis of PMA phenotype.
To our best knowledge, this is the first study, which demonstrates that PMA phenotype is characterized by a significantly higher degree of airway hyperresponsiveness in luteal phase of the menstrual cycle than other asthma phenotypes. This is consistent with the results of study by Tan et al. which revealed that airway responsiveness to AMP in asthmatic women was highest during the premenstrual period (13). However, in this study authors did not analyze the degree of bronchial hyperresponsiveness (BHR) in relation to the presence of cyclical perimenstrual asthma exacerbations. A higher degree of BHR in the luteal phase of the menstrual cycle in PMA women is likely related to different, than observed in other asthma phenotypes, absolute concentrations (or concentration ratio) or greater variability of female sex hormones levels. Previously performed studies show that estrogen and progesterone receptors are widely distributed in the lower airways (15) and both hormones are involved in modifying airway responsiveness (14). Hellings et al. revealed that progesterone increased BHR in a mouse model of allergic asthma (17). On the contrary, estradiol has been shown to reduce BHR by increasing the activity of acetylcholine esterase in bronchial epithelium nerve endings (18) and estrogen replacement therapy in murine model of allergic asthma decreased airway hyperresponsiveness to metacholine in a dose-dependent way (19). Detailed explanation of the impact of the distribution of female sex hormones on the severity of BHR in PMA asthmatics certainly requires further studies. However, our study for the first time highlights the possibility that higher perimenstrual BHR degree in women with PMA (when compared to non-PMA asthmatics) may be responsible for more severe exacerbations and often poorer response to treatment, which, according to many authors (5-7), are characteristic for this phenotype.
The results of some former studies suggested, that PMA seems to be closely linked to some atopy markers. In their study Pereira-Vega et al. proved that women with PMA had total blood IgE values increased above normal (20). Authors however, did not compare total IgE concentrations between PMA and non-PMA asthmatics. In our study, inter-group comparisons revealed, that women with PMA had significantly higher total IgE serum concentration (when compared to non-PMA asthmatics) which reversely correlated with the PC20 value in the bronchial challenge test with metacholine. Given the fact, that women with parasitic diseases and any other chronic diseases, in which total serum IgE is increased above normal values, have been excluded from the study, the obtained results indicate, that PMA phenotype is more strongly associated with atopic mechanisms than other asthma phenotypes. Another possible explanation of these results would be, that in some asthmatic women high propensity to atopy (associated with the increased bronchial reactivity) overlaps with substantial fluctuations in female sex hormones levels during perimenstrual period, leading to a further increase in the degree of BHR, known as perimenstrual asthma.
In our study we demonstrated, that in PMA subjects sputum concentrations of both, eotaxin and IL-4 during luteal phase of the menstrual cycle were higher than in non-PMA asthmatics. To our best knowledge, this is the first study which assessed markers of inflammation in lower airways of PMA subjects and compared them with other asthma phenotypes. To date, a number of studies have stressed that recruitment and activation of inflammatory cells in asthma is controlled by the release of chemotactic agents, such as eotaxin (32). In a recent meta-analysis (33) increased concentrations of eotaxin have been reported in blood and sputum of asthmatic patients when compared to healthy controls. Sputum eotaxin levels in that study were significantly elevated in unstable (versus stable) asthma patients suggesting, that this chemokine might be a useful biomarker for the assessment of asthma severity and control. Increased sputum eotaxin level in PMA patients during luteal phase of the cycle in our study is in agreement with the results of the above-mentioned meta-analysis (33) proving once again, that higher concentrations of eotaxin may be associated with the more severe disease course. The increased sputum IL-4 concentration in PMA group found in our study is interesting, especially in the light of the co-existing significantly higher total IgE serum concentration in these patients. It has previously been proved, that in order for a B lymphocyte to switch to IgE synthesis, a signal provided by a Th2 cell in the form of the IL-4/IL-13 is necessary (34). The results of our study not only suggest, that PMA phenotype is associated with the increased IgE synthesis (when compared to other asthma phenotypes) but also with the IL-4 up-regulation in the lower airways of these patients.
Conclusions
Patients with perimenstrual asthma are characterized by a significantly higher degree of bronchial hyperreactivity and higher concentrations of total serum IgE, when compared with non-PMA asthmatics. PC20 to metacholine and total serum IgE in these patients are reversely correlated. Higher degree of bronchial hyperreactivity in patients with PMA (as compared to non-PMA asthmatics) is associated with increased concentration of eotaxin and IL-4 in lower airways of these patients suggesting a shift in the type-1/type-2 cytokine balance toward a type-2 response.
Acknowledgements
Funding: The research was supported by the Polish National Science Centre [5979/B/P01/2011/40]. There was no industrial sponsorship.
Footnote
Conflicts of Interest: The results of the study were presented during the European Respiratory Society in Munich (Germany), 6-10.09.2014 (Eur Respir J Suppl 2014;44:2).
Ethical Statement: The study was approved by Ethics Committee of the Medical Faculty, Silesian Medical University, Katowice, Poland and it conforms to the provisions of in accordance with the Helsinki Declaration as revised in 2013. The informed, written consent was obtained from all study participants.
References
- Rao CK, Moore CG, Bleecker E, et al. Characteristics of perimenstrual asthma and its relation to asthma severity and control: data from the Severe Asthma Research Program. Chest 2013;143:984-92. [Crossref] [PubMed]
- Murphy VE, Gibson PG. Premenstrual asthma: prevalence, cycle-to-cycle variability and relationship to oral contraceptive use and menstrual symptoms. J Asthma 2008;45:696-704. [Crossref] [PubMed]
- Dratva J, Schindler C, Curjuric I, et al. Perimenstrual increase in bronchial hyperreactivity in premenopausal women: results from the population-based SAPALDIA 2 cohort. J Allergy Clin Immunol 2010;125:823-9. [Crossref] [PubMed]
- Sabry EY. Relation of perimenstrual asthma with disease severity and other allergic co-morbidities--the first report of perimenstrual asthma prevalence in Saudi Arabia. Allergol Immunopathol (Madr) 2011;39:23-6. [Crossref] [PubMed]
- Suzuki K, Hasegawa T, Sakagami T, et al. Analysis of perimenstrual asthma based on questionnaire surveys in Japan. Allergol Int 2007;56:249-55. [Crossref] [PubMed]
- Eliasson O, Scherzer HH, DeGraff AC Jr. Morbidity in asthma in relation to the menstrual cycle. J Allergy Clin Immunol 1986;77:87-94. [Crossref] [PubMed]
- Lenoir RJ. Severe acute asthma and the menstrual cycle. Anaesthesia 1987;42:1287-90. [Crossref] [PubMed]
- Pauli BD, Reid RL, Munt PW, et al. Influence of the menstrual cycle on airway function in asthmatic and normal subjects. Am Rev Respir Dis 1989;140:358-62. [Crossref] [PubMed]
- Vrieze A, Postma DS, Kerstjens HA. Perimenstrual asthma: a syndrome without known cause or cure. J Allergy Clin Immunol 2003;112:271-82. [Crossref] [PubMed]
- Zierau O, Zenclussen AC, Jensen F. Role of female sex hormones, estradiol and progesterone, in mast cell behavior. Front Immunol 2012;3:169. [Crossref] [PubMed]
- Nakasato H, Ohrui T, Sekizawa K, et al. Prevention of severe premenstrual asthma attacks by leukotriene receptor antagonist. J Allergy Clin Immunol 1999;104:585-8. [Crossref] [PubMed]
- Mirdal GM, Petersson B, Weeke B, et al. Asthma and menstruation: the relationship between psychological and bronchial hyperreactivity. Br J Med Psychol 1998;71:47-55. [Crossref] [PubMed]
- Tan KS, McFarlane LC, Lipworth BJ. Loss of normal cyclical beta 2 adrenoceptor regulation and increased premenstrual responsiveness to adenosine monophosphate in stable female asthmatic patients. Thorax 1997;52:608-11. [Crossref] [PubMed]
- Haggerty CL, Ness RB, Kelsey S, et al. The impact of estrogen and progesterone on asthma. Ann Allergy Asthma Immunol 2003;90:284-91; quiz 291-3, 347.
- Couse JF, Lindzey J, Grandien K, et al. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology 1997;138:4613-21. [Crossref] [PubMed]
- Tan KS, McFarlane LC, Coutie WJ, et al. Effects of exogenous female sex-steroid hormones on lymphocyte beta 2-adrenoceptors in normal females. Br J Clin Pharmacol 1996;41:414-6. [Crossref] [PubMed]
- Hellings PW, Vandekerckhove P, Claeys R, et al. Progesterone increases airway eosinophilia and hyper-responsiveness in a murine model of allergic asthma. Clin Exp Allergy 2003;33:1457-63. [Crossref] [PubMed]
- Degano B, Prévost MC, Berger P, et al. Estradiol decreases the acetylcholine-elicited airway reactivity in ovariectomized rats through an increase in epithelial acetylcholinesterase activity. Am J Respir Crit Care Med 2001;164:1849-54. [Crossref] [PubMed]
- Dimitropoulou C, Drakopanagiotakis F, Chatterjee A, et al. Estrogen replacement therapy prevents airway dysfunction in a murine model of allergen-induced asthma. Lung 2009;187:116-27. [Crossref] [PubMed]
- Pereira-Vega A, Sánchez JL, Maldonado JA, et al. Premenstrual asthma and atopy markers. Ann Allergy Asthma Immunol 2010;105:218-22. [Crossref] [PubMed]
- Yamauchi K. Airway inflammatory marker. Nihon Rinsho 2001;59:1938-44. [PubMed]
- Barczyk A, Pierzchala W, Sozañska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med 2003;97:726-33. [Crossref] [PubMed]
- Peters MC, Mekonnen ZK, Yuan S, et al. Measures of gene expression in sputum cells can identify TH2-high and TH2-low subtypes of asthma. J Allergy Clin Immunol 2014;133:388-94. [Crossref] [PubMed]
- de Oliveira AP, Peron JP, Damazo AS, et al. Female sex hormones mediate the allergic lung reaction by regulating the release of inflammatory mediators and the expression of lung E-selectin in rats. Respir Res 2010;11:115. [Crossref] [PubMed]
- Oguzulgen IK, Turktas H, Erbas D. Airway inflammation in premenstrual asthma. J Asthma 2002;39:517-22. [Crossref] [PubMed]
- Miyaura H, Iwata M. Direct and indirect inhibition of Th1 development by progesterone and glucocorticoids. J Immunol 2002;168:1087-94. [Crossref] [PubMed]
- 2017 GINA Report, Global Strategy for Asthma Management and Prevention. Available online: http://ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention/
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [Crossref] [PubMed]
- Hargreave FE, Sterk P, Adelroth EC, et al. Airway responsiveness to histamine or methacholine: advances in measurement and interpretation. Respiration 1986;50 Suppl 2:72-6. [Crossref] [PubMed]
- Semik-Orzech A, Barczyk A, Wiaderkiewicz R, et al. Interleukin 17 and RANTES levels in induced sputum of patients with allergic rhinitis after a single nasal allergen challenge. Ann Allergy Asthma Immunol 2009;103:418-24. [Crossref] [PubMed]
- Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol 2004;113:59-65. [Crossref] [PubMed]
- Lampinen M, Carlson M, Håkansson LD, et al. Cytokine-regulated accumulation of eosinophils in inflammatory disease. Allergy 2004;59:793-805. [Crossref] [PubMed]
- Wu D, Zhou J, Bi H, et al. CCL11 as a potential diagnostic marker for asthma? J Asthma 2014;51:847-54. [Crossref] [PubMed]
- Poulsen LK, Hummelshoj L. Triggers of IgE class switching and allergy development. Ann Med 2007;39:440-56. [Crossref] [PubMed]


