Effects of cryopreservation on tracheal allograft antigenicity in dogs
Introduction
Tracheal allografts may be used in surgical reconstruction after long segmental tracheal resections. Rose and colleagues [1979] reported the first clinically attempted tracheal transplantation, citing promising short-term (9-week) results (1). Similar accounts surfaced thereafter, detailing the use of allografts for reconstructing lengthy tracheal defects (>5 cm) in humans (2-4).
Cryopreservation of various allografts, including skin, heart valves, and arteries, has been amply investigated over the years. Although some data suggest a decline in allograft antigenicity by doing so, thus raising hopes of immunosuppressant-free transplantation (and eliminating side effects, costs) (5-7), there is also evidence to the contrary (8-11). Yokomise and colleagues successfully conducted tracheal allotransplantation in dogs, without use of immunosuppressants, by immersing grafts in a cryopreservative solution and deep freezing (−85 °C) for 285±28 days (~8–10 months) (12). Aside from one animal sacrificed for histologic study, the canine recipients of such transplants survived for more than 2 months. The longest observation period at time of publication was 293 days. A variety of similar experiments in rats, rabbits, dogs, and even nonhuman primates have since ensued (13-16).
Yokomise and associates found that tracheal epithelium, being the primary source of major histocompatibility complex (MHC) antigens, was depleted in all allografts after cryopreservation for 285±28 days. Thus, reduced antigenicity was considered the basis for the benefits derived in tracheal allografts (12). However, it was unclear how the above specified period of cryopreservation was selected, raising further issues on how prolonged cryopreservation actually alters the tracheal epithelium or what duration of storage is required to fully denude mucosa for transplantation with impunity (i.e., immunosuppressant-free). Some reports have indicated that allograft epithelium is not always exfoliated after periods of cryopreservation (13,17,18), disputing initial data. Hence, the impact of cryopreservation on epithelial shedding and antigenicity in tracheal allografts has yet to be fully elucidated.
For this study, the same cryopreservation method devised by Yokomise was implemented, transplanting variably cryopreserved (1–10 months) tracheal allografts into dogs, without use of immunosuppressants. Our aim was to characterize the epithelial changes in these allografts after cryopreservation and analyze experimental results after orthotopic transplantation, thus determining the effect of cryopreservation on tracheal allograft antigenicity.
Methods
Eight mongrel adult dogs served as donor dogs and fifty mongrel adult dogs as experimental animals in this study.
Cryopreservation of tracheal allografts
Our cryopreservation method was the same as that of Yokomise et al. (12). In brief, the entire trachea of a donor dog (sacrificed) was harvested and trimmed into several segments (7–8 rings each). The segments were then placed in 50-mL sterile tubes filled with cryopreservative solution, containing Dulbecco’s modified Eagle medium, 10% dimethyl sulfoxide (DMSO), 20% fetal calf serum and 0.1 mmol/L trehalose, for subsequent deep-freeze storage (−85 °C, 1–10 months).
Histologic examination
After cryopreservation (1–10 months), tracheal grafts were thawed in an incubator (37 °C) for 15 min and rinsed (10 times) in physiologic saline solution, all in original storage tubes. Before transplantation, one ring was resected from each end of the tracheal grafts for routine processing. Tracheal samples were fixed for 24 h in 10% neutral buffered formalin solution at room temperature. After fixation, the samples were washed in distilled water, dehydrated in graded alcohol, embedded in paraffin, and sectioned at 4-µm thickness. Sections were then stained with hematoxylin and eosin (H&E). In some grafts, the condition of tracheal epithelium was checked after thawing but before rinsing. Serial H&E-stained sections of two tracheal grafts cryopreserved for 5–6 months were also examined. MHC-II antigen was detected immunohistochemically in 5-µm frozen sections, using a labeled streptavidin biotin method. The primary antibody (1:50 dilution) was rat anti-canine MHC-II antibody [MCA 1044; Serotec (Bio-Rad), Oxford, UK], and the secondary antibody (1:300 dilution) was a biotin-conjugated rabbit anti-rat antibody (STAR52; Serotecc). Each section was incubated (room temperature, 1 h) sequentially with first primary and then secondary antibody. All sections were observed under an optical microscope (Olympus BX41, Tokyo, Japan).
Tracheal allotransplantation
Test animals were stratified as recipients of allografts cryopreserved for either 1–7 months (group 1, n=9, shorter than the report of Yokomise et al.) or for 8–10 months (group 2, n=6, the same as that of Yokomise et al.). After intramuscular injection (ketamine hydrochloride, 10 mg/kg; xylazine hydrochloride, 4 mg/kg) for narcosis, anesthesia (oxygen, 50%; nitrous oxide, 50%; halothane, 1%) was maintained via oral endotracheal intubation. Orthotopic tracheal transplantation began with recipient supine dogs, raising an omental pedicle graft in each through midline abdominal incision for transdiaphragmatic right chest placement. Each dog was then kept in left lateral decubitus position for right chest entry through fourth intercostal space. Five rings of intrathoracic trachea were subsequently resected, inserting a sterile flexible intubation tube into distal cut end to maintain intraoperative ventilation and anesthesia. Five corresponding rings of tracheal allograft (cryopreserved, thawed, and rinsed according to protocol) were ultimately transplanted in situ by telescopic anastomosis, using continuous 4-0 prolene sutures (Ethicon, Inc., Somerville, NJ, USA) to join proximal and distal cut ends of recipient trachea to allograft. At completion, the previously inserted tube was removed, returning respiration to transoral intubation. Anastomotic sites and allograft were both covered by omental pedicle.
Postoperative management and observation
In this experiment, no immunosuppressants were administered to either group. A dual antibiotic regimen (ampicillin sodium and cloxacillin sodium, each 250 mg/day) was given intramuscularly during postoperative week 1 and then orally until the end of week 4. Under general anesthesia (ketamine hydrochloride, 10 mg/kg; xylazine hydrochloride, 4 mg/kg) via intramuscular route, luminal surfaces of tracheal allografts were examined by flexible bronchoscopy, done weekly during postoperative weeks 2–4 and then monthly until animals died or were euthanized. Degree of tracheal stricture was designated as follows: less than 30% reduction of lumen, slight; 30–60% reduction, moderate; and more than 60% reduction, severe. Upon death or sacrifice of animals, allografts were removed for routine H&E tissue staining and light microscopy.
All animals have received humane care in compliance with the 1996 “Guide for the Care and Use of Laboratory Animals” as recommended by the US National Institutes of Health (NIH).
Results
Histologic evaluation after cryopreservation
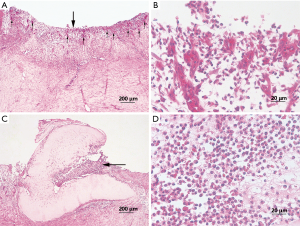
In both groups, the epithelial linings of tracheal allografts tended to exfoliate after cryopreservation, with patchy loss and sparse cilia in microscopic views; but overall, the epithelium of most tracheal grafts cryopreserved for 1–10 months was largely intact (Figure 1). Rinsing of grafts in physiologic saline solution (10 times) had no major effect on epithelial surfaces. Sporadically, however, graft epithelium did partially exfoliate or detach after rinsing (Figure 2). MHC-II positivity was demonstrable in immunostained sections of cryopreserved grafts (Figure 3). In serial sections of grafts cryopreserved for 5 and 6 months, respectively, epithelial loss occurred to some degree at the edge area and partial margins were even denuded, but mid-portions were essentially intact (Figure 4).
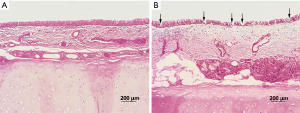
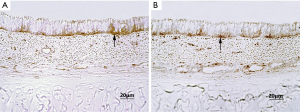
Bronchoscopic and macroscopic findings in surviving animals
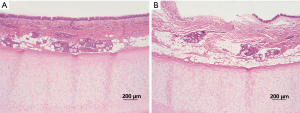
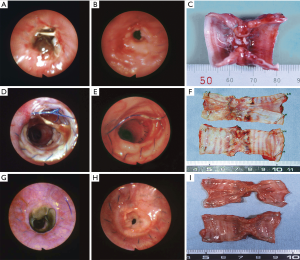
No animal deaths resulted from complications of transplant surgery (Table 1). In group 1, eight animals were sacrificed or died within 50 days postoperatively. Bronchoscopic evaluations revealed the development of lethal graft strictures, created by proliferating granulation tissue (Figure 5A,B). Grossly, the tracheal allografts were shrunken and severely narrowed (Figure 5C). Although one dog suffered only slight stricture, surviving for 38 months until sacrificed (Figure 5D,E), the graft was still notably atrophic (Figure 5F). In group 2, survival times of recipient dogs were somewhat longer, ranging from 28–126 days, with three animals surviving for >60 days. However, severe strictures were observed by bronchoscopy in all dogs of group 2 (Figure 5G,H); and when ultimately sacrificed, macroscopic findings were similar to those of group 1 (Figure 5I).
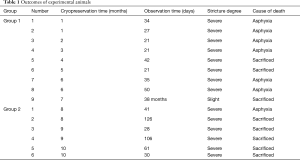
Full table
Histologic evaluation of grafts after death or euthanasia
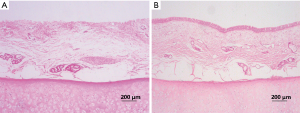
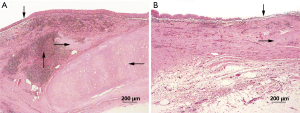
In microscopic sections, the majority of luminal surfaces in harvested grafts of all dogs (both groups) appeared bare, except in one animal observed for 38 months. Intense infiltrates of lymphocytes and monocytes were observed in submucosa. Entire allografts displayed fibroblastic proliferation, rich in neocapillaries, regardless of cryopreservation period (Figure 6A,B). Allograft cartilage also appeared partly absorbed or destroyed, accompanied by severe mixed infiltrates of granulocytes, lymphocytes, and monocytes (Figure 6C,D). In the sole remaining animal (group 1, dog 9), histologic examination showed severe lymphocytic/monocytic infiltration and cartilage absorption involving the entire graft, especially near anastomotic sites, with essentially intact epithelium (Figure 7A). At the mid-section, a monolayer of epithelium lined the surface (Figure 7B).
Discussion
Immunologic rejection is a major drawback of allotransplantation, requiring immunosuppressant agents to maintain the structural and functional integrity of grafted tissues. Based on a considerable body of evidence, it is the antigenicity of tracheal allografts that mandates intervention after transplantation to control the immune response (14,19). Indeed, when Yokomise and colleagues performed fresh tracheal transplantation in five mongrel dogs (20), without immunosuppressive treatment, all of the animals died from severe tracheal stenosis (i.e., rejection) within 1 month. In a previous experiment of ours, fresh tracheal allotransplantation alone (with no immunosuppression) likewise prompted immune rejection, causing asphyxia death of recipients due to granulation tissue proliferation, luminal stenosis, and allograft necrosis (21). Given the facts above, fresh tracheal allotransplantation was not performed as normal controls in compliance with the principle of reduction alternatives to animal experiments. Yokomise et al. reported that the epithelium of the tracheal allografts was depleted completely after long-term cryopreservation (~8–10 months) (12). For this reason, the long-term (8–10 months) cryopreserved tracheal grafts served as references in the present study. Furthermore, we wondered when the epithelial depletion would happen after cryopreservation for 1–7 months. Therefore, the tracheal grafts that cryopreserved for 1–7 months were assigned to experimental group (group 1).
On the other hand, the trachea is simple in structure and function, relative to other organs (kidney, liver, or heart). Luminal patency is ensured by its cartilaginous core, and its epithelial lining serves in expelling of mucous. Its antigenicity is also considered weak by comparison (1,22). Bujia and colleagues identified MHC-II (positive in mucosa; negative in cartilage) as a determining factor in tracheal immune rejection after transplantation (23). Unlike other organs, the distribution of MHC in tracheal allografts facilitates elimination of antigenicity. We have demonstrated in a related study that eradication of luminal epithelium from tracheal allografts greatly diminishes their antigenicity in the context of immunosuppressant-free allotransplantation (21). On this basis, immune rejection should not be life-threatening or an allotransplant jeopardized, if the cartilage of such tracheal preparations remains viable and luminal patency is maintained (22).
Generally, cryopreservation helps maintain the stability of organs and tissues, including histologic attributes, viability, and biomechanical properties. However, the potential for cryopreservation to reduce allograft antigenicity remains controversial. Some studies suggest that cryopreservation not only preserves harvested tissues (e.g., skin, heart valves, and arteries) for a period of time, but antigenicity (and thus immune rejection) is also mitigated through this means (5-7). Still, the mechanism by which cryopreservation reduces allograft antigenicity is not entirely clear, with some available evidence supporting the opposite premise. Clarke and coworkers replaced aortic roots in 47 children with cryopreserved aortic valve allografts but found results unsatisfactory. They blamed allograft failures on immune rejection possibly due to retained antigenicity after cryopreservation (8). Moriyama and colleagues also compared inflammatory infiltrates after heterotopic transplantation of fresh and cryopreserved arterial allografts to gauge any suppression of antigenicity through cryopreservation, reaching a negative conclusion (9). Using rats to assess the effects of cryopreservation on heart valve and vascular allografts, Saito et al. similarly discovered that cell viability was sustained and allograft antigenicity was unaltered during cryopreservation (10). Finally, by characterizing both class I and class II antigenicity states of cryopreserved skin, Tomita and associates concluded that cryopreservation had no effect on skin allograft antigenicity (11). Hence, the impact of cryopreservation on allograft antigenicity remains uncertain to date.
A team led by Yokomisehas nevertheless observed both cartilaginous viability and mucosal denudation in tracheal allografts subjected to cryopreservation (−85 °C) for 285±28 days (~8–10 months). This particular method was credited with a presumptive decline in tracheal allograft antigenicity (12). Such reasoning is corroborated by another of our earlier studies in which the antigenicity of tracheal allografts was reduced by detergent-induced stripping of epithelium (21). However, Mukaida and colleagues have documented luminal epithelium on tracheal allografts 10 days postoperatively, confirming via modified polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) analysis that such epithelial residuals were identical in phenotype to donor peripheral blood samples (17). Nakanishi and associates have also noted that the epithelium of cryopreserved allografts did not appear denuded upon thawing (18). It is therefore apparent that despite cryopreservation, epithelial cells in tracheal allografts are capable of survival. Furthermore, an investigation of immune rejection by Stoelben et al., using cryopreserved tracheal transplants in a standardized heterotopic rat model, showed obstructive luminal connective tissue in allografts, indicating no significant loss of allograft antigenicity (13).
Some evidence has indicated that the blood supply would be critical if successful tracheal transplantation were ever possible. Only a vascularized graft can fulfill the anatomic mechanical and anti-infectious functions of the trachea (24). Thus, in this study, we used the omental pedicle to support the blood supply. We showed the thawed tracheal epithelium had a tendency to progressively exfoliate, and cilia were sparse. However, the linings of most allografts were generally intact, and epithelial MHC-II positivity was confirmed by immunostaining. Our unpublished data has verified that even after 2–4 years of cryopreservation, the ciliated epithelium of tracheal allografts persists in essence, as does MHC-II expression. All animals (but one) in both groups that were sacrificed or dead within 126 days of transplantation had developed lethal strictures. Survival times were also somewhat prolonged by extending the allograft cryopreservation period. Overall, animals of group 2 survived longer than those of group 1, implying a relative benefit for long-term cryopreservation by continued weakening of antigenicity. Upon sacrifice, histologic preparations of all test grafts revealed completely denuded luminal surfaces, with fibroblastic proliferation, extensive neocapillary ingrowth, intense lymphocytic/monocytic infiltrates, and cartilaginous absorption. Although a single animal of group 1 suffered only slight stricture during 38 months of observation, this was not interpreted as transplantation success. The graft was grossly shrunken. Microscopically, the ciliated pseudostratified columnar epithelium was evident, severely infiltrated by lymphocytes and monocytes, and the cartilaginous rings were nearly gone. In this instance, the epithelial component was likely critical in preventing allograft stricture by prohibiting fibroblastic proliferation (25).
For our purposes, the Yokomise cryopreservation method was adopted, including the same storage solution and temperature (−85 °C). Although our outcomes differed, there are certain reasons for disparities. First of all, individual differences in experimental technique may be a factor. As we have demonstrated, the epithelium of thawed grafts was exfoliated in places, with sparse cilia. In some grafts, such patches were more apparent after rinsing than before; and in specific grafts, the epithelium was almost completely denuded after rinsing. Preoperative management after cryopreservation may also be nuanced, depending upon the experimenter involved. Distinctions in thawing duration and in rinsing duration, frequency, and intensity may affect epithelial status. Therefore, any stripping of tracheal epithelium during the course of cryopreservation is subject to procedures used for thawing and rinsing.
Second, in the Yokomise’s report and in the present study, seven to eight rings of donor trachea were cryopreserved, reserving five rings for transplantation. A sampling from each ring of a tracheal graft would better depict its epithelial status throughout the entire graft, although this was not feasible. Tissue specimens were subsequently restricted to both ends of tracheal grafts. In addition, serial sections of grafts undergoing 5 and 6 months, respectively of cryopreservation were also examined, showing denuded patches at partial margins, and generally intact epithelium at mid-portions. The prevailing conditions at graft margins, being particularly vulnerable during thawing/rinsing procedures, may not be fully representative of each graft. This perhaps explains the microscopic evidence of denuded epithelium in grafts, which nevertheless developed lethal strictures after transplantation.
Third, an appropriate space was chosen for frozen storage of grafts to maintain a steady temperature and avoid interferences. In some instances, it was difficult to avoid freeze-thaw activity long-term in tracheal grafts grouped with other materials for ongoing experiments. Multiple freeze-thaw cycles alone may promote epithelial loss.
As with other organs, tracheal transplantation is also hampered by donor shortages. Cryopreservation can alleviate this problem, but not without some tissue damage. In a related experiment, we investigated the ultrastructural changes of cryopreserved tracheal rings in rats. As cryopreservation time increased, ultrastructural injury to tracheal chondrocytes became more pronounced (26). In particular, we consider tracheal epithelial separation as one manifestation of thermal damage. In our view, however, such exposures do not result in completely denuded surfaces, so residual epithelium would remain to incite post-transplantation rejection. A decellularization process, such as detergent treatment or combined detergent-enzyme incubation would seem more effective than cryopreservation to eliminate antigen-laden tracheal graft epithelium (21,27). Future research should focus more on issues of thermal damage and the viability of long-term cryopreserved tracheal grafts. It may be that vitrification is a better method to preserve tracheal grafts (28).
In summary, tracheal allograft epithelium tended to exfoliate somewhat after cryopreservation but was retained in most instances. Hence, epithelial surfaces were not regularly denuded by cryopreservation. The residual epithelium retained antigenicity, with a capacity for immune rejection, leading to transplantation failures. Extending the cryopreservation period did not eliminate the antigenicity of allografts. Present findings indicate that the reduced antigenicity of tracheal allografts achieved through cryopreservation is insufficient to prevent fatal rejection.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethics Committee of Zhongshan Hospital, Fudan University (No. 2013-254).
References
- Rose KG, Sesterhenn K, Wustrow F. Tracheal allotransplantation in man. Lancet 1979;1:433. [Crossref] [PubMed]
- Levashov , Yu N, Yablonsky PK, Cherny SM, et al. One-stage allotransplantation of thoracic segment of the trachea in a patient with idiopathic fibrosing mediastinitis and marked tracheal stenosis. Eur J Cardiothorac Surg 1993;7:383-6. [Crossref] [PubMed]
- Delaere P, Vranckx J, Verleden G, et al. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N Engl J Med 2010;362:138-45. [Crossref] [PubMed]
- Xu L, Zhang S, Li J, et al. Human tracheal allotransplant with greater omentum for revascularization. Exp Clin Transplant 2014;12:448-53. [PubMed]
- Hirasé Y, Kojima T, Takeishi M, et al. Transplantation of long-term cryopreserved allocutaneous tissue by skin graft or microsurgical anastomosis: experimental studies in the rat. Plast Reconstr Surg 1993;91:492-501. [Crossref] [PubMed]
- Oei FB, Stegmann AP, Vaessen LM, et al. Immunological aspects of fresh and cryopreserved aortic valve transplantation in rats. Ann Thorac Surg 2001;71:S379-84. [Crossref] [PubMed]
- Solanes N, Rigol M, Castella M, et al. Cryopreservation alters antigenicity of allografts in a porcine model of transplant vasculopathy. Transplant Proc 2004;36:3288-94. [Crossref] [PubMed]
- Clarke DR, Campbell DN, Hayward AR, et al. Degeneration of aortic valve allografts in young recipients. J Thorac Cardiovasc Surg 1993;105:934-41; discussion 41-2. [PubMed]
- Moriyama S, Utoh J, Sun LB, et al. Antigenicity of cryopreserved arterial allografts: comparison with fresh and glutaraldehyde treated grafts. ASAIO J 2001;47:202-5. [Crossref] [PubMed]
- Saito A, Motomura N, Kakimi K, et al. Cryopreservation does not alter the allogenicity and development of vasculopathy in post-transplant rat aortas. Cryobiology 2006;52:251-60. [Crossref] [PubMed]
- Tomita Y, Zhang QW, Yoshikawa M, et al. Lack of effect of cryopreservation on the class I and class II antigenicities of skin allografts. Transplant Proc 1998;30:60-2. [Crossref] [PubMed]
- Yokomise H, Inui K, Wada H, et al. Long-term cryopreservation can prevent rejection of canine tracheal allografts with preservation of graft viability. J Thorac Cardiovasc Surg 1996;111:930-4. [Crossref] [PubMed]
- Stoelben E, Harpering H, Haberstroh J, et al. Heterotopic transplantation of cryopreserved tracheae in a rat model. Eur J Cardiothorac Surg 2003;23:15-20. [Crossref] [PubMed]
- Hisamatsu C, Maeda K, Tanaka H, et al. Transplantation of the cryopreserved tracheal allograft in growing rabbits: effect of immunosuppressant. Pediatr Surg Int 2006;22:881-5. [Crossref] [PubMed]
- Iyikesici T, Tuncozgur B, Sanli M, et al. Two-piece cryopreserved tracheal allotransplantation: an experimental study. Eur J Cardiothorac Surg 2009;36:722-6. [Crossref] [PubMed]
- Murakawa T, Nakajima J, Motomura N, et al. Successful allotransplantation of cryopreserved tracheal grafts with preservation of the pars membranacea in nonhuman primates. J Thorac Cardiovasc Surg 2002;123:153-60. [Crossref] [PubMed]
- Mukaida T, Shimizu N, Aoe M, et al. Origin of regenerated epithelium in cryopreserved tracheal allotransplantation. Ann Thorac Surg 1998;66:205-8. [Crossref] [PubMed]
- Nakanishi R, Hashimoto M, Muranaka H, et al. Effect of cryopreservation period on rat tracheal allografts. J Heart Lung Transplant 2001;20:1010-5. [Crossref] [PubMed]
- Pérez D, Cano JR, Quevedo S, et al. Effects of deep hypothermic preservation on posttransplant viability of tracheal grafts. Transplant Proc 2010;42:3244-6. [Crossref] [PubMed]
- Yokomise H, Inui K, Wada H, et al. High-dose irradiation prevents rejection of canine tracheal allografts. J Thorac Cardiovasc Surg 1994;107:1391-7. [PubMed]
- Liu Y, Nakamura T, Yamamoto Y, et al. Immunosuppressant-free allotransplantation of the trachea: the antigenicity of tracheal grafts can be reduced by removing the epithelium and mixed glands from the graft by detergent treatment. J Thorac Cardiovasc Surg 2000;120:108-14. [Crossref] [PubMed]
- Liu Y, Nakamura T, Shimizu Y, et al. Tracheal allotransplantation in beagle dogs without immunosuppressants. Ann Thorac Surg 2001;72:1190-4. [Crossref] [PubMed]
- Bujía J, Wilmes E, Hammer C, et al. Class II antigenicity of human cartilage: relevance to the use of homologous cartilage graft for reconstructive surgery. Ann Plast Surg 1991;26:541-3. [Crossref] [PubMed]
- Grillo HC. Tracheal replacement: a critical review. Ann Thorac Surg 2002;73:1995-2004. [Crossref] [PubMed]
- Hysi I, Kipnis E, Fayoux P, et al. Successful orthotopic transplantation of short tracheal segments without immunosuppressive therapy. Eur J Cardiothorac Surg 2015;47:e54-61. [Crossref] [PubMed]
- Liu Y, Yang Y, Ding J, et al. Ultrastructural changes in cryopreserved tracheal grafts of sprague-dawley rats. ASAIO J 2009;55:509-13. [Crossref] [PubMed]
- Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. Lancet 2008;372:2023-30. [Crossref] [PubMed]
- Xu H, Shi HC, Zang WF, et al. An experimental research on cryopreserving rabbit trachea by vitrification. Cryobiology 2009;58:225-31. [Crossref] [PubMed]


