Pediatric lung transplantation
Lung transplants in children have been undertaken since the 1980s, and nowadays, pediatric lung transplantation is considered as an accepted therapy option in carefully selected children with end-stage parenchymal and vascular pulmonary diseases, providing the well-selected pediatric candidate a net survival benefit and improved health-related quality of life (1-4).
The 2016 International Society for Heart and Lung Transplantation (ISHLT) Thoracic Transplant Registry Report shows that over 100 pediatric lung transplants per annum were reported to the Registry worldwide (5).
Since set-up of the ISHLT Pediatric Thoracic Transplant Registry, more than 2,000 pediatric lung transplant procedures have been reported in total to date; however, less than 30 centres carry out such procedures in children, the majority of centers perform less than 5 procedures per year (5). In Europe, pediatric lung transplants are done in predominantly in adult centers, with variable input of pediatricians. Despite this, very good results were achieved in pediatric lung transplantation as reported by individual adult centers (6). On the other hand, published data indicated that not only transplant center volume, but also specific pediatric expertise effects outcome of pediatric lung transplantation (7). Further, a recent analysis of United Network for Organ Sharing (UNOS) data analysis including more than 2,000 patients across 67 transplant centers revealed that particularly CF-specific expertise in cystic fibrosis (CF), the most common primary indication for pediatric lung transplantation, predicts better long-term outcome of lung transplantation for CF (7).
Nevertheless it is vital to comprehend that children undergoing lung transplantation present a challenge as children are not ‘just small adults’. The surgical approach can be more challenging, and effects of immunosuppression in the developing immune system of a child, and psychosocial aspects, particularly in adolescents, have to be taken into consideration (2).
Primary indications for lung transplant, referral and transplant evaluation
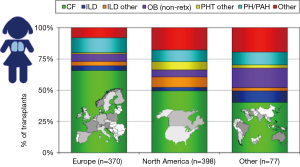
Indications for lung transplants in children are different compared to adults. In adults, the most frequent primary indication for lung transplantation is chronic obstructive pulmonary disease (COPD) with one-third of all procedures, followed by interstitial lung disease (ILD) and CF as described elsewhere. In children, the overall leading diagnosis for lung transplantation is end-stage CF pulmonary disease; nevertheless, indications vary by age. In children less than 1 year of age, congenital heart disease (CHD) is the most frequent underlying disease. In children aged 1 to 10 years of age, end-stage CF pulmonary disease and idiopathic pulmonary arterial hypertension (IPAH) are the most frequent primary indications for lung transplants. In older children and adolescents, CF is most common (5). It is important to emphasize that there are regional differences worldwide regarding primary indications leading to referral for pediatric lung transplantation, almost certainly reflecting diverse practices for referral, varying disease management and organ allocation rules for pediatric lung transplant candidates (5). In North America, half of pediatric lung transplants are carried out in children with end-stage pulmonary CF. In Europe on the other hand, over two-thirds of children undergoing lung transplantation suffer with CF (5) (Figure 1).
In children, lung re-transplants are rarely performed. Over the last two decades, only 100 pediatric lung re-transplant have been reported to the ISHLT Thoracic Transplant Registry, predominately from North America (8). Re-transplantation is predominantly undertaken in older children and adolescents and more frequently carried out beyond the first 12 months after primary transplantation. In most children, chronic lung allograft dysfunction (CLAD), primarily bronchiolitis obliterans syndrome (BOS), is the underlying cause leading to lung re-transplants (8).
Pediatric heart-lung transplantation is very rarely performed nowadays, with less than 10 procedures per year reported to the ISHLT Thoracic Transplant Registry in recent years (9). Heart-lung transplants in children are only performed in very few centers. The vast majority of pediatric heart-lung transplant procedures are undertaken in children with IPAH, in a minority in those children suffering with CHD (9).
In general, all children with end-stage parenchymal and vascular pulmonary diseases on maximal medical treatment should be referred to a transplant program for lung transplant assessment if the predicted life expectancy is below 2 years (2,4). On the whole, the predicted life expectancy without lung transplantation has to be balanced with the expected post-transplant survival, taking into account the potential time on a waiting list, which could be particularly longer for children due to the general lack of suitable smaller donor organs. In Europe, children would most likely be referred to an adult transplant center with pediatric experience for transplant evaluation, in North America, generally to a pediatric transplant program. In order to maximize the net survival benefit of lung transplantation as the ultimate therapy option in children with advanced pulmonary disease, careful candidate selection is absolutely critical (2,4). The ISHLT Pulmonary Council has recently published an update on guidelines for referral and selection of lung transplant candidates, for the first time ever, including a general guidance on the pediatric lung transplant candidate selection (10). All in all it is important to note that no prospective, randomized studies have been conducted to date to support the published guidelines. Overall, timing of referral to a transplant center is similar in adult and pediatric practice, even though younger children should ideally be referred early as long waiting times for suitable smaller donor organs are to be expected (2,4). The updated ISHLT consensus document includes disease-specific criteria for referral and listing for transplant of the most common primary indications for lung transplantation. As CF is the most common pathology leading to pediatric lung transplantation and referral patterns and listing criteria are similar to adults, the consensus recommendations are discussed here in more detail. Patients with end-stage CF pulmonary disease should be referred for lung transplant assessment if on maximal medical therapy and in case of a forced expiratory volume in one second (FEV1) <30% predicted, a 6-minute walk distance (6MWD) <400 m, pulmonary hypertension (outside a hypoxic exacerbation period) with a mean pulmonary arterial pressure (PAP) >25 mmHg measured invasively by right heart catheterization or a systolic PAP >35 mmHg on echocardiography, or other clinical signs of end-stage CF pulmonary disease such as a poor recovery from exacerbations, pneumothorax, life-threatening hemoptysis, or acute respiratory failure needing non-invasive ventilation (NIV) (10). Maximal medical therapy in patients with CF should ideally include a trial of newer CF therapies based on recent but advances in the field of CF research, therapies that have recently been introduced to the market modulating the basic defect in CF (11). Recent studies in highly selected CF patients using such disease modulators (potentiators/correctors) have shown promising clinical results, even in patients with advanced CF pulmonary disease or patients already listed for lung transplant (12). Therefore, patients with CF undergoing lung transplant evaluation should ideally be assessed for their eligibility of such CF-disease modulating therapies depending on their CF genotype. In selected lung transplant candidates, such disease modulating agents might lead to clinical stabilization, ideally prolonging the time of listing for transplant or stabilizing patients on the waiting list, all of which should aid to maximize net survival in CF patients.
In general, particularly young underweight females with CF and rapid pulmonary function decline should be referred early as this subgroup of CF patients has a poor prognosis. Then again, underweight body habitus itself may not in generally have a significant negative impact on survival of pediatric CF patients undergoing lung transplantation according to a recent ISHLT Thoracic Transplant Registry data analysis including over 800 pediatric lung transplant recipients (13). Listing of patients with CF for lung transplant is generally recommended in case of respiratory failure with hypoxia alone (PaO2 <8 kPa or <60 mmHg) or hypercapnia (PaCO2 >6.6 kPa or >50 mmHg), if requiring long-term NIV, rapid pulmonary function decline, frequent hospitalizations and/or WHO functional class IV (10).
At the assessment of every pediatric lung transplant candidate, the child and family require to be appropriately informed and sufficiently educated. Even a child should be willing to commit to the planned transplant operation and to generally consent to the close post-operative long-term follow-up needed. Child and family support is vital and should be implemented prior to listing for transplantation if not already set up (2,4).
Overall, adherence to medical treatment needs to be evaluated prior to listing for lung transplantation. Non-adherence is a leading cause for the development of CLAD and inferior long-term outcome post-transplant, in particular in adolescents, a well-known features following transplantation across all solid organ types (14).
As a general rule, contraindications in pediatric lung transplantation are similar to adult practice, but relative contraindications might be different between centers (4). In CF lung transplant candidates particularly acceptance of listing for lung transplant may differ among transplant programs, depending on CF airway pathogens isolated prior to transplantation. Nevertheless, all transplant candidates have to be carefully assessed in view of potential infection risks (15). For CF transplant patients, some transplant programs have shown that patients chronically infected with Burkholderia cenocepacia have poorer post-transplant outcomes, but patients chronically infected with Burkholderia cepacia complex species other than B. cenocepacia do frequently have post-transplant survival comparable to other CF lung transplant recipients (16-19). Other airway important pathogens, such as nontuberculous mycobacteria (NTM) are frequently isolated in CF patients (up to 20%) referred for lung transplantation (20,21). Isolation of NTM in the airways of CF patients should not be considered an absolute contraindication for lung transplantation; however, all NTMs need to be classified first. In case of isolation of M. abscessus and depending on the antibiotic resistance pattern, some centers would consider such circumstance a contraindication for lung transplant. Nevertheless, appropriate treatment strategies needs to be discussed and implemented with support by the transplant infectious diseases specialist.
In pediatric lung transplant candidates with CF, the extra-pulmonary manifestations of CF have to be looked at carefully. In case of advanced CF-related liver disease, combined lung-liver transplantation needs to be evaluated. Other common extra-pulmonary disease manifestations of CF include diabetes mellitus, chronic rhino-sinusitis, CF-related bone disease and bowel problems such as recurrent episodes of distal intestinal obstruction syndrome (DIOS) (4).
Mechanical ventilation before lung transplantation has until recently been considered a contraindication for lung transplant in the majority of pediatric centers due to poor results previously reported (22). But newer data on the use of extracorporeal life/lung support (ECLS) prior to pediatric lung transplantation—particularly in awake patients—illustrate that pre-transplant ECLS might not generally lead to poor post-transplant outcomes if children are selected very carefully and treated at experienced centers (23-25).
Often smaller children wait a long time on the waiting list before a suitable donor organ is allocated, and in such candidates ECLS as a bridge to lung transplantation is to be considered. Children should be fully evaluated and already listed candidates on the transplant waiting list with rapidly advancing respiratory failure, to stabilize the child until a suitable donor organ is allocated. In general, candidates for ECLS as bridge to transplantation should be in single-organ failure with a good rehabilitation potential. The recently published consensus document by the ISLHT Pulmonary Council lists contraindication for ECLS as bridge to transplantation such as septic shock and multi-organ failure that are also applicable for pediatric candidates (10). A larger analysis of pediatric UNOS data on the use of ECMO at the time of lung transplantation showed no negative impact on the post-operative mortality rate (26). Further, newly published positive outcome results from Australia and Switzerland in small pediatric patient cohorts would promote the use of ECLS as a “bridge strategy” in highly selected, ideally awake, children (6,27). If pediatric patients are kept “awake” on ECLS prior to lung transplantation, physical deconditioning can be prevented as children have the possibility to get mobilized and perform physiotherapy, supporting post-operative recovery and rehabilitation (28-30). Thus, pre-transplant ECLS in children is nowadays considered as superior alternative to long-time mechanical ventilation by most centers, taking potential complications of the two methods into consideration. Nevertheless, more outcome data on pediatric lung transplant candidates on ECLS are needed to figure out key factors associated with poor post-operative results.
The technical details of the various forms of ECLS and possible complications using ECLS are beyond the scope of this chapter.
Donor acceptability criteria in pediatric lung transplantation
The ISHLT published donor acceptability criteria in the past, predominantly based on the adult lung transplant experience (31). The major limit of lung transplantation is the worldwide lack of suitable donor organs. Strategies to address the shortage of donor lungs include usage of so-called “marginal” donor organs (or extended criteria donor organs), organ donation after circulatory death (DCD) with or without ex-vivo lung perfusion (EVLP) as graft preparation pre-operatively, and graft size reduction (i.e., lobar lung transplants) (32,33). The latter is of particular interest in pediatric lung transplant candidates due to the shortage of donor organs for smaller children (34). Donor-recipient (D/R) size mismatch is an important aspect with a considerable influence on post-operative results (35-37). In oversized allografts, complications include atelectasis and segmental (or sub-segmental) airway distortion with impaired mucus clearance, a potential source of recurrent infections. Undersized allografts potentially cause persistent pneumothorax, graft hyperextension, compromised vital lung capacity with reduced hemodynamic reserve, higher likelihood of primary graft dysfunction and the development of CLAD (38,39). In one of the largest pediatric data sets, the Zurich Group report on outcomes of 20 out of 29 children and adolescents after lung transplantation using size-reduced donor lungs due to pre-operative size-mismatches (40). No significant different short- and mid-term survival between the “full-size” and the “size-reduced bilateral transplant” patients were found.
Various centers described surgical techniques to successfully perform size-reduction of donor lung grafts in children; details are beyond the scope of this chapter. The Zurich Group also published a case report of a simultaneously performed bilateral lobar lung transplant derived from one donor into two small adolescents with CF (41). Both patients have an unimpaired lung function even more than five years post-transplant with no evidence of CLAD. However, such an approach is not routinely performed to overcome donor shortage in smaller lung transplant recipients.
Management of pediatric lung transplant recipients and post-transplant outcome
Immunosuppressive treatment is the keystone to prevent lung allograft rejection (42). In general, the majority of children undergoing lung transplantation receive induction therapy, most commonly an interleukin-2 receptor antagonist (5,43). Similar to adults, children generally are on maintenance triple immunosuppression post-transplant (cyclosporine/tacrolimus, mycophenolate mofetil, steroids). Nowadays, tacrolimus is more commonly prescribed than cyclosporine. Pediatric programs cooperating within the International Pediatric Lung Transplant Collaborative (IPLTC) have recently agreed upon an immunosuppressant treatment protocol for children undergoing lung transplantation that includes tacrolimus, mycophenolate mofetil and prednisolone (Samuel Goldfarb, personal communication).
Although graft function is to be preserved, immunosuppressant-related side effects are common in children following lung transplantation. Therefore, strategies need to be put into standard practice to reduce immunosuppression-related side effects such as nephrotoxicity through careful therapeutic drug monitoring and lowering of target levels of calcineurin-inhibitors, to avoid acute-reversible and chronic-irreversible renal impairment (44). Instead of a “one fits all approach”, tailored immunosuppression and a personalized therapy approach is to be advocated, particularly in children with a good lung allograft function and no evidence of CLAD (4).
Further, infectious complications are common causes for morbidity and mortality in pediatric lung transplant recipients, accounting for almost 50% of death during the first twelve months after transplantation (5). Children following lung transplantation that are at a high risk for infections caused by cytomegalovirus (CMV)—defined as positive recipient or donor serology—get CMV prophylaxis (45). However, current practice among pediatric programs varies, even though international consensus guidelines—but not specifically for children—on the management of CMV in solid organ transplant recipients were published (46). Furthermore, fungal lung infections are not uncommon, and recently published pediatric data show a decreased 12-month post-transplant survival (47). Respiratory viral infections are very common after lung transplantation in children, sources are often siblings or peer groups and associated with a decreased 1-year survival (48). In order to at least reduce the burden of vaccine preventable diseases, children should be vaccinated prior to be placed on the transplant waiting list. It is well known that vaccinations are frequently incomplete before transplantation in these children. National vaccination guidelines should be followed; vaccinations guidelines for pediatric lung transplant patients are center-specific, no consensus guidelines exist to the author’s knowledge. Vaccinations of household contacts are also highly recommended (49).
Overall, survival after pediatric lung transplantation is comparable to adults (5,50). As in adult lung transplantation, survival following pediatric lung transplantation has improved over time, primarily due to superior early post-operative survival, i.e., better surgical techniques and early post-operative intensive care management (2,4).
According to the most recent ISHLT Thoracic Transplant Registry Report, 5-year survival after lung was 53% in the recent era [2002–2009] (5). Individual pediatric programs have published survival data exceeding the results of the ISHLT Registry (6). The Zurich Group recently put out its contemporary data on lung transplantation in children and adolescents (up to 20 years of age), quoting an overall 5-year survival over 75% in predominantly patients with CF (6). However, it remains somewhat difficult to predict survival following lung transplantation in children with advanced CF pulmonary disease. Various studies in adults have been published demonstrating a survival benefit in adult lung transplantation for CF. But in children with CF, survival data following lung transplantation are mostly based on single-center reports (51,52). However, a report from Zurich clearly illustrates a true survival benefit in 80 CF patients undergoing lung transplantation, with no negative impact of pediatric age (<18 years of age) on post-transplant survival (53). In the study by Hofer et al., estimated 5-year survival without transplant was 33% compared to a 5-year post-transplant survival of 67%. On the other hand, Liou and co-workers concluded based on UNOS data that lung transplantation would not improve survival in children with CF (54). Further, Liou and co-workers published a controversially discussed analysis in 2007 using US Cystic Fibrosis Foundation Patient Registry and Organ Procurement and Transplantation Network (OPTN) data on patients undergoing lung transplantation between 1992 and 2002 to demonstrate that only five of over 500 CF children awaiting lung transplantation can expect an improved survival (55). Various authors discussed future directions of studies investigating survival benefit of children with CF undergoing lung transplantation following publication of the study by Liou et al.; however, data are still lacking (56,57).
As in adult lung transplantation, the development of CLAD is the major hurdle to achieve better overall survival also after lung transplantation in children. BOS, the most common form of CLAD, is the leading cause of death (>40%) by 5 years following transplantation. Similar to adults, further, around half of the surviving children develop BOS by 5 years after lung transplantation (5). On the other hand, overall functional status of children surviving lung transplantation is reported to be good.
As described elsewhere in detail, international clinical practice guidelines for the diagnosis and management of BOS have recently been published (58). To date, no well-proven therapy approach exists to successfully manage CLAD in lung transplant recipients, both in adults and children. Similar attempts include change/augmentation of immunosuppression, and use of macrolides, extracorporeal photopheresis (ECP), and total lymphoid irradiation (TLI) (59). Pediatric data do generally not exist. The final option for advanced lung allograft failure is lung re-transplantation. As described above, data on pediatric lung re-transplantation is limited, most cases performed for CLAD-BOS, predominantly in older children (8). Based on limited published outcome data, pediatric lung re-transplants appear to be more successful if re-transplantation follows a minimum of twelve months after primary transplantation and in children not mechanically ventilated at time of re-transplantation (60). Ideally, pediatric candidates for lung re-transplantation should have no second organ failure.
Lastly, with improvements in pediatric lung transplantation detailed above, transition from pediatric to adult care is more frequent. As transition is not a single event but rather a process of an adolescent lung transplant recipient being transferred to adult care providers, varies aspects of other transitions of an adolescent that take place simultaneously need to be looked at (i.e., autonomy, self-identity, cognition, sexuality, physical appearance, education) (61). Ideally, transition should advocate self-care and decision-making of the adolescent, but also include parents/caregivers, taking into account the adolescent’s chronological age, physical and cognitive maturity. In general, transition is regarded of interest for both pediatric and adult transplant care teams (2,4).
To sum up, pediatric lung transplants have successfully been carried out in children of all age groups, including infants, with encouraging outcomes. Similar to adult lung transplantation, the development of CLAD remains the burden of lung transplantation in children and adults restricting long-term success. Potential pediatric candidates for lung transplantation should be referred early, assessed thoroughly and selected very carefully in order to maximize the overall net survival benefit following pediatric lung transplantation. Specific pediatric aspects of lung transplantation are the shortage of suitable donor organs for smaller children and psychosocial aspects and adherence, in particular, in adolescents.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Mendeloff EN. The history of pediatric heart and lung transplantation. Pediatr Transplant 2002;6:270-9. [Crossref] [PubMed]
- Benden C. Specific aspects of children and adolescents undergoing lung transplantation. Curr Opin Organ Transplant 2012;17:509-14. [Crossref] [PubMed]
- Hayes D Jr, Benden C, Sweet SC, et al. Current state of pediatric lung transplantation. Lung 2015;193:629-37. [Crossref] [PubMed]
- Schmid FA, Benden C. Special considerations for the use of lung transplantation in pediatrics. Expert Rev Respir Med 2016;10:655-62. [Crossref] [PubMed]
- Goldfarb SB, Levvey BJ, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Nineteenth pediatric lung and heart-lung transplantation report – 2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant 2016;35:1196-205. [Crossref] [PubMed]
- Schmid FA, Inci I, Bürgi U, et al. Favorable outcome of children and adolescents undergoing lung transplantation at a European adult center in the new era. Pediatr Pulmonol 2016;51:1222-8. [Crossref] [PubMed]
- Hayes D Jr, Sweet SC, Benden C, et al. Transplant center volume and outcomes in lung transplantation for cystic fibrosis. Transpl Int 2017;30:371-7. [Crossref] [PubMed]
- Benden C, Goldfarb SB, Edwards LB, et al. The rregistry of the International Society for Heart and Lung Transplantation: seventeenth official pediatric lung and heart-lung transplantation report – 2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:1025-33. [Crossref] [PubMed]
- Benden C, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: fifteenth pediatric lung and heart-lung transplantation report – 2012. J Heart Lung Transplant 2012;31:1087-95. [Crossref] [PubMed]
- Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014 – an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2015;34:1-15. [Crossref] [PubMed]
- Bosch B, De Boeck K. Searching for a cure for cystic fibrosis. A 25-year quest in a nutshell. Eur J Pediatr 2016;175:1-8. [Crossref] [PubMed]
- Elborn JS, Ramsey BW, Boyle MP, et al. Efficacy and safety of lumacaftor/ivacaftor combination therapy in patients with cystic fibrosis homozygous for Phe508del CFTR by pulmonary function subgroup: a pooled analysis. Lancet Respir Med 2016;4:617-26. [Crossref] [PubMed]
- Benden C, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Sixteenth Official Pediatric Lung and Heart-Lung Transplantation Report – 2013; focus theme: age. J Heart Lung Transplant 2013;32:989-97. [Crossref] [PubMed]
- Simons LE, Blount RL. Identifying barriers to medication adherence in adolescent transplant recipients. J Pediatr Psychol 2007;32:831-44. [Crossref] [PubMed]
- Luong ML, Morrissey O, Husain S. Assessment of infection risks prior to lung transplantation. Curr Opin Infect Dis 2010;23:578-83. [Crossref] [PubMed]
- Murray S, Charbeneau J, Marshall BC, et al. Impact of burkholderia infection on lung transplantation in cystic fibrosis. Am J Respir Crit Care Med 2008;178:363-71. [Crossref] [PubMed]
- Alexander BD, Petzold EW, Reller LB, et al. Survival after lung transplantation of cystic fibrosis patients infected with Burkholderia cepacia complex. Am J Transplant 2008;8:1025-30. [Crossref] [PubMed]
- Hopkins PM, Kidd TJ, Coulter C, et al. Death after lung transplantation in cystic fibrosis patients infected with Burkholderia cepacia. Am J Respir Crit Care Med 2009;179:257-8. [Crossref] [PubMed]
- De Soyza A, Meachery G, Hester KL, et al. Lung transplantation for patients with cystic fibrosis and Burkholderia cepacia complex infection: a single-center experience. J Heart Lung Transplant 2010;29:1395-404. [Crossref] [PubMed]
- Olivier KN, Weber DJ, Wallace RJ, et al. Nontuberculous mycobacteria. I: multicenter prevalence study in cystic fibrosis. Am J Respir Crit Care Med 2003;167:828-34. [Crossref] [PubMed]
- Chalermskulrat W, Sood N, Neuringer IP, et al. Non-tuberculous mycobacteria in end stage cystic fibrosis: implications for lung transplantation. Thorax 2006;61:507-13. [Crossref] [PubMed]
- Elizur A, Sweet SC, Huddleston CB, et al. Pre-transplant mechanical ventilation increases short-term morbidity and mortality in pediatric patients with cystic fibrosis. J Heart Lung Transplant 2007;26:127-31. [Crossref] [PubMed]
- Cypel M, Keshavjee S. Extracorporeal life support as a bridge to lung transplantation. Clin Chest Med 2011;32:245-51. [Crossref] [PubMed]
- Hoopes CW, Kukreja J, Golden J, et al. Extracorporeal membrane oxygenation as a bridge to pulmonary transplantation. J Thorac Cardiovasc Surg 2013;145:862-7. [Crossref] [PubMed]
- Inci I, Klinzing S, Schneiter D, et al. Outcome of extracorporeal membrane oxygenation as a bridge to lung transplantation: An institutional experience and literature review. Transplantation 2015;99:1667-71. [Crossref] [PubMed]
- Hayes D Jr, McConnell PI, Tobias JD, et al. Survival in children on extracorporeal membrane oxygenation at the time of lung transplantation. Pediatr Transplant 2015;19:87-93. [Crossref] [PubMed]
- Casswell GK, Pilcher DV, Martin RS, et al. Buying time: The use of extracorporeal membrane oxygenation as a bridge to lung transplantation in pediatric patients. Pediatr Transplant 2013;17:E182-8. [Crossref] [PubMed]
- Olsson KM, Simon A, Strueber M, et al. Extracorporeal membrane oxygenation in nonintubated patients as bridge to lung transplantation. Am J Transplant 2010;10:2173-8. [Crossref] [PubMed]
- Garcia JP, Iacono A, Kon ZN, et al. Ambulatory extracorporeal membrane oxygenation: a new approach for bridge-to-lung transplantation. J Thorac Cardiovasc Surg 2010;139:e137-9. [Crossref] [PubMed]
- Schmidt F, Sasse M, Boehne M, et al. Concept of "awake venovenous extracorporeal membrane oxygenation" in pediatric patients awaiting lung transplantation. Pediatr Transplant 2013;17:224-30. [Crossref] [PubMed]
- Orens JB, Boehler A, de Perrot M, et al. A review of lung transplant donor acceptability criteria. J Heart Lung Transplant 2003;22:1183-200. [Crossref] [PubMed]
- Aigner C, Winkler G, Jaksch P, et al. Size-reduced lung transplantation: an advanced operative strategy to alleviate donor organ shortage. Transplant Proc 2004;36:2801-5. [Crossref] [PubMed]
- Santos F, Lama R, Alvarez A, et al. Pulmonary tailoring and lobar transplantation to overcome size disparities in lung transplantation. Transplant Proc 2005;37:1526-9. [Crossref] [PubMed]
- Mueller C, Hansen G, Ballmann M, et al. Size reduction of donor organs in pediatric lung transplantation. Pediatr Transplant 2010;14:364-8. [Crossref] [PubMed]
- Inci I, Schuurmans MM, Kestenholz P, et al. Long-term outcomes of bilateral lobar lung transplantation. Eur J Cardiothorac Surg 2013;43:1220-5. [Crossref] [PubMed]
- Aigner C, Mazhar S, Jaksch P, et al. Lobar transplantation, split lung transplantation and peripheral segmental resection – reliable procedures for downsizing donor lungs. Eur J Cardiothorac Surg 2004;25:179-83. [Crossref] [PubMed]
- Eberlein M, Reed RM, Permutt S, et al. Parameters of donor-recipient size mismatch and survival after bilateral lung transplantation. J Heart Lung Transplant 2012;31:1207-13.e7. [Crossref] [PubMed]
- Eberlein M, Permutt S, Chahla MF, et al. Lung size mismatch in bilateral lung transplantation is associated with allograft function and bronchiolitis obliterans syndrome. Chest 2012;141:451-60. [Crossref] [PubMed]
- Eberlein M, Reed RM, Bolukbas S, et al. Lung size mismatch and primary graft dysfunction after bilateral lung transplantation. J Heart Lung Transplant 2015;34:233-40. [Crossref] [PubMed]
- Benden C, Inci I, Weder W, et al. Size-reduced lung transplantation in children – an option worth to consider. Pediatr Transplant 2010;14:529-33. [Crossref] [PubMed]
- Inci I, Benden C, Kestenholz P, et al. Simultaneous bilateral lobar lung transplantation: one donor serves two recipients. Ann Thorac Surg 2013;96:e69-71. [Crossref] [PubMed]
- Coelho T, Tredger M, Dhawan A. Current status of immunosuppressive agents for solid organ transplantation in children. Pediatr Transplant 2012;16:106-22. [Crossref] [PubMed]
- Hayes D Jr, Kirkby S, Wehr AM, et al. A contemporary analysis of induction immunosuppression in pediatric lung transplant recipients. Transpl Int 2014;27:211-8. [Crossref] [PubMed]
- Sarwal M, Pascual J. Immunosuppression minimization in pediatric transplantation. Am J Transplant 2007;7:2227-35. [Crossref] [PubMed]
- Danziger-Isakov LA, Worley S, Michaels MG, et al. The risk, prevention, and outcome of cytomegalovirus after pediatric lung transplantation. Transplantation 2009;87:1541-8. [Crossref] [PubMed]
- Kotton CN, Kumar D, Caliendo AM, et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation 2010;89:779-95. [Crossref] [PubMed]
- Liu M, Worley S, Mallory GB Jr, et al. Fungal infections in pediatric lung transplant recipients: colonization and invasive disease. J Heart Lung Transplant 2009;28:1226-30. [Crossref] [PubMed]
- Liu M, Worley S, Arrigain S, et al. Respiratory viral infections within one year after pediatric lung transplant. Transpl Infect Dis 2009;11:304-12. [Crossref] [PubMed]
- Benden C, Danziger-Isakov LA, Astor T, et al. Variability in immunization guidelines in children before and after lung transplantation. Pediatr Transplant 2007;11:882-7. [Crossref] [PubMed]
- Zafar F, Heinle JS, Schecter MG, et al. Two decades of pediatric lung transplant in the United States: have we improved? J Thorac Cardiovasc Surg 2011;141:828-32. [Crossref] [PubMed]
- Görler H, Strüber M, Ballmann M, et al. Lung and heart-lung transplantation in children and adolescents: a long-term single center experience. J Heart Lung Transplant 2009;28:243-8. [Crossref] [PubMed]
- Gruber S, Eiwegger T, Nachbaur E, et al. Lung transplantation in children and young adults: a 20 years single center experience. Eur Respir J 2012;40:462-9. [Crossref] [PubMed]
- Hofer M, Benden C, Inci I, et al. True survival benefit of lung transplantation for cystic fibrosis patients: the Zurich experience. J Heart Lung Transplant 2009;28:334-9. [Crossref] [PubMed]
- Liou TG, Adler FR, Huang D. Use of lung transplantation survival models to refine patient selection in cystic fibrosis. Am J Respir Crit Care Med 2005;171:1053-9. [Crossref] [PubMed]
- Liou TG, Adler FR, Cox DR, et al. Lung transplantation and survival in children with cystic fibrosis. N Engl J Med 2007;357:2143-52. [Crossref] [PubMed]
- Sweet SC, Aurora P, Benden C, et al. Lung transplantation and survival in children with cystic fibrosis: solid statistics – flawed interpretation. Pediatr Transplant 2008;12:129-36. [Crossref] [PubMed]
- Sweet SC, Benden C, Elidemir O. Lung transplantation and survival in children with cystic fibrosis. N Engl J Med 2008;358:1754. [PubMed]
- Meyer KC, Raghu G, Verleden GM, et al. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J 2014;44:1479-503. [Crossref] [PubMed]
- Benden C, Danziger-Isakov L, Faro A. New developments in treatment after lung transplantation. Curr Pharm Des 2012;18:737-46. [Crossref] [PubMed]
- Scully BB, Zafar F, Schecter MG, et al. Lung retransplantation in children: appropriate when selectively applied. Ann Thorac Surg 2011;91:574-9. [Crossref] [PubMed]
- LaRosa C, Glah C, Baluarte HJ, et al. Solid-organ transplantation in childhood: transitioning to adult health care. Pediatrics 2011;127:742-53. [Crossref] [PubMed]


