Does BioGlue contribute to anastomotic pseudoaneurysm after thoracic aortic surgery?
Introduction
Perioperative bleeding in aortic surgical procedures is a major complication that causes significant morbidity and mortality (1). Re-exploration for bleeding has been reported to be a predictor of early mortality following aortic surgery and is associated with increased risk of postoperative myocardial infarction and renal failure. Several surgical adhesives have been developed to improve hemostasis by reinforcing suture lines and repairing fragile tissues (2-5). One useful surgical adhesive is bovine serum albumin-glutaraldehyde glue (BSAG, i.e., BioGlue®, CryoLife, Kennesaw, GA, USA), an agent composed of 45% purified bovine serum albumin and 10% glutaraldehyde. The two solutions mix completely within the delivery tip during application and begin to polymerize immediately, reaching full bonding strength within 2 min (6). Glutaraldehyde binds with bovine serum albumin and tissue proteins at the repair site, creating a mechanical seal independent of the body’s coagulation cascade (6). BioGlue was approved for use in cardiovascular surgery by the FDA in 2001 (7).
Although several animal studies and clinical reports have illuminated the benefits of using BioGlue in aortic surgical operations over the past 15 years (8-11), some reports suggest that BioGlue may severely injure the aortic tissue, ultimately leading to re-dissection or pseudoaneurysm formation at the anastomotic sites (1,9,10,12-16). This study increases the patient number of an earlier pilot study we published previously (14) and aims to examine whether the use of BioGlue is associated with a high incidence of anastomotic pseudoaneurysm formation following surgical repair of thoracic aortic disease.
Methods
The Human Investigation Committee of Yale University approved this retrospective study (HIC # 1509016418).
The database of the Aortic Institute at Yale-New Haven Hospital was reviewed to identify patients in whom BioGlue was used during their aortic surgical procedures between July 2001 and May 2015.
Our policy has been to use BioGlue only in cases of acute aortic dissection or extreme tissue fragility. BioGlue was used sparingly between the dissected layers or at the anastomosis between vascular prosthesis and the friable aortic tissues. Only a thin, uniform layer was applied to maximize the bonding strength and to avoid bulky residue and adverse events. Our technique for using BioGlue has previously been described in detail (17). All our aortic/graft anastomoses were done with reinforcement with an external Teflon strip. For acute dissections, we applied BioGlue between dissected layers supported by internal and external felt strips. At discharge, patients were recommended to have regular imaging studies (computed tomography or magnetic resonance imaging, CT or MRI) 6 weeks postoperatively and every 1 to 2 years thereafter.
Clinical follow-up was based on a review of medical records, office visits, and phone calls to patients or their relatives or physicians. The follow-up CT or MR scans and radiology reports were specifically reviewed by our team and confirmed by the senior author to detect anastomotic pseudoaneurysm formation. For patients who had multiple CT scans, we used the most recent CT scan that was available in the analysis.
Statistical analysis was performed using SPSS for Windows version 16.0 (SPSS Inc, Chicago, IL, USA). Data were expressed as the mean ± standard deviation (or median, 25–75% IQR) or number (percentage) and compared using the Student t (or Mann-Whitney U) test for continuous variables and Pearson χ2 or Fisher exact probability test for categorical variables. All statistical tests were 2-sided and a P value of less than 0.05 was considered statistically significant.
Results
A total of 233 patients with BioGlue use were identified with a mean age of 63.5±14.0 years (median 66; range 14–88 years; 25–75% IQR 54–74). There were 149 males (63.9%) and 84 females (36.1%). Bicuspid aortic valve was present in 46 patients (19.7%) and connective tissue disorders in 5 (2.1%). During this time period, 1,060 patients underwent surgery for non-dissection, “degenerative” thoracic aortic aneurysms at our institution. Among them, BioGlue was not used in 891 (84.1%). The senior author (John A. Elefteriades) performed approximately 99% of these procedures.
Indications and procedures
Emergent or urgent aortic surgical repair was performed in 68 cases (29.2%). The indication for surgery was thoracic aortic aneurysm in 169 (72.5%) patients, aortic dissection in 49 (21.0%), intramural hematoma in 9 (3.9%), penetrating aortic ulcer in 3 (1.3%), pseudoaneurysm in 2 (0.8%) and coarctation in 1 (0.4%). Ascending aortic repair was performed in 189 cases (81.1%), which included ascending aortic with hemiarch repair in 96 cases (41.2%) and ascending aortic with total arch repair in 31 (13.3%). Isolated arch repair was performed in 10 patients (4.3%) and descending aortic repair in 34 (14.6%).
Early outcomes
Operative death (defined as all deaths occurring during the same hospitalization in which the operation was performed even if after 30 days or after discharge but within 30 postoperative days) occurred in 17 patients (7.3%). The mortality rates were 10.2% (7/68) and 6.1% (10/165) for emergency and elective repair (P=0.274), and 11.8% (4/34) and 6.3% (12/189) for descending and ascending aorta repair, respectively. Causes of death included ventricular fibrillation in 6 patients (2.6%), stroke in 5 (2.1%), low cardiac output in 3 (1.3%), bleeding in 2 (0.8%) and respiratory failure in 1 (0.4%). Re-exploration for bleeding was required in 24 (10.3%) cases. Neurologic complications occurred in 22 cases in 19 patients (9.0%, some multiple), including stroke in 9 (3.9%), paralysis in 6 (2.6%), transient ischemic attack in 4 (1.7%), and coma/encephalopathy in 3 (1.3%).
Although patients with aortic dissection (and intramural hematoma and penetrating ulcer) had significantly higher rates of emergency operation (70.7% vs. 14.2%, P<0.001) and neurological complications (15.5% vs. 5.9%, P=0.030) compared to patients with aortic aneurysms, the incidences of mortality (12.1% vs. 5.3%, P=0.132) and re-exploration for bleeding (13.8% vs. 8.9%) did not differ significantly between two groups (Table 1).

Full table
Late imaging follow-up
Clinical follow-up was complete in 100% (216/216) for a mean duration of 5.8±4.3 years (median 5.3 years; 25–75% IQR 1.7–9.8). As shown in Table 2, follow-up CT or MR scan was available in 177 of 216 hospital survivors (81.9%). The mean duration of imaging follow-up was 2.4 years (median 0.6 years; range 5 days to 12.9 years; 25–75% IQR 0.2–3.6 years).

Full table
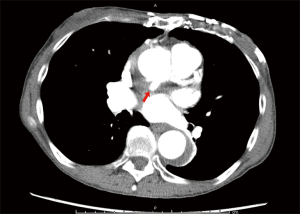
Of the 177 CT or MR scans available, anastomotic pseudoaneurysm was detected in only one patient (0.6%). This patient was a cachectic, extremely frail elderly female who, 6 years previously, underwent aortic valve replacement with a mechanical prosthesis. She underwent reoperative replacement of the ascending aorta and deep hemiarch with a 24-mm Hemashield® (Maquet, Rastatt, Germany) graft under deep hypothermic arrest for repair of a dissecting aneurysm that involved the ascending aorta and transverse arch. At 3 years postoperatively, follow-up CT angiogram detected a pseudoaneurysm located at the ascending aortic anastomosis (Figure 1). She remained asymptomatic and was managed medically. No further intervention was performed.
Discussion
The clinical safety and benefits of using BioGlue in thoracic aortic surgery, including repair of aortic dissections and aortic root and arch reconstructions, have been well documented. In a prospective multicenter clinical trial, BioGlue was used as an adjunct to standard repair of aortic tissues in comparison with a standard surgical repair (7). A significant decrease in anastomotic bleeding (P<0.001) and pledget use (P=0.047) was seen in the BioGlue group as compared with the control group. Fehrenbacher and associates observed a mortality rate of 3.3% in 92 aortic surgical procedures using BioGlue as an adjunct for anastomotic hemostasis, with 28.3% of patients requiring no blood transfusions (9).
However, with its increased use, long-term complications of using BioGlue have been reported, including late anastomotic pseudoaneurysm formation in small or large case series (Table 3). Reports on reoperations for pseudoaneurysms have described necrotic, fibrosed, and excessively thinned aortic tissue found at the site of adhesive application (1,9,10,12,14,15,18,21,23). In 2001, Kazui reported a case of pseudoaneurysm at 5 months after initial repair of acute dissection (18). Ngaage and associates reported a second case of pseudoaneurysm formation at 2 years after repair of type A dissection (23). In the series of Fehrenbacher and associates, 3 of 92 patients (3.3%) required reoperations for pseudoaneurysm (9). David and colleagues also reported the pathologic findings in 3 patients who underwent reoperations for pseudoaneurysm (13). Besides its theoretical direct tissue toxicity, preclinical studies by Fürst and colleagues proposed that polymerized BioGlue may potentially release glutaraldehyde, causing cytotoxic effects to adjacent tissues by inducing inflammation, edema formation, and necrosis at the site of application (24). In other published preclinical studies, the same effects as reported by Fürst have not been observed (25,26). Additionally, Downing suggests in a letter that the use of surgical glue allows more patients to survive surgery, and those patients who, without glue would have died, now present with later complications (21).

Full table
Though there are multiple reported cases, late pseudoaneurysm formation did not uniformly relate to the use of BioGlue in all previous studies. In multiple, large single center reports (8-10,14,22), pseudoaneurysm rates were well within the expected range for these disease states, which has been reported to be as high as 6.0% (27,28). In a series of 95 type A dissections in which late pseudoaneurysm occurred in some patients treated with GRF glue, Westaby and colleagues noted that this complication was not associated with BioGlue (29). In the series by Bavaria and associates, there were no instances of pseudoaneurysm in 58 patients up to 38 months (30). In our previous study of 97 patients who were followed up for a mean of 15 months after aortic surgery, we found two cases of pseudoaneurysm that were likely unrelated to the use of BioGlue, which were located remote from the surgical site (14). While all these series were relatively small and lacked longer follow-up, the rates of pseudoaneurysm formation observed did not exceed what is reported in the literature with standard surgical repair, as shown in Table 3.
In the present study, we seek to directly address the issue if the use of BioGlue is associated with a high incidence of anastomotic pseudoaneurysm after surgical repair of thoracic aortic disease. This study has the advantages of a larger sample size (233 patients) and longer follow-up (2.4 years on average). Our experience, primarily by a single surgeon well experienced with proper BioGlue techniques, has shown that use of BioGlue in this group of patients is not associated with a high incidence of anastomotic pseudoaneurysm formation following surgical repair of thoracic aortic disease.
However, given the documented potential risk, we do not recommend the use of BioGlue for routine thoracic aortic surgical procedures. In our practice, we have only utilized BioGlue in cases of acute aortic dissection or severe tissue fragility for two reasons: (I) we do not feel BioGlue is necessary for hemostasis in the vast majority of patients, and (II) we respect the caution raised in the literature (9,20,21,23) regarding the potential contribution of BioGlue to false aneurysm formation. Rather, in certain circumstances, especially in cases of acute aortic dissection or extreme tissue fragility, the benefits of using BioGlue truly outweigh potential risks. For these patients, the use of BioGlue should not be discouraged on the basis of the potential risk of late anastomotic pseudoaneurysm formation. It is important to note that BioGlue is not a substitute for proper suturing technique. Proper application of BioGlue, including applying small, even amounts to complete suture lines, is critical to good outcomes with minimized complications.
Study limitations
Even with a larger patient cohort and longer mean follow-up, this study is limited in that it is retrospective in nature and does not include a concurrent control group. Nor did we compare the use of BioGlue with any other surgical adhesives. However, the incidence of pseudoaneurysm in this series is so low (0.6%), it is hard to imagine a lower rate in any control group. A contributor to the low incidence of pseudoaneurysm formation may have been the standardized surgical techniques employed, as the senior author performed 99% of operations. Despite our best efforts, we were still unable to obtain 100% radiologic follow-up. However, we know with certainty that there was no concerning rate of pseudoaneurysm in the many patients for whom scans were available. Another concern pertains to the wide time range of imaging follow-up, as a narrower range of time may be preferable from a methodological perspective. Nevertheless, the sample size of 233 patients and 177 CT scans at a mean follow-up of 2.4 years are sufficient for the nature and purpose of this study.
Conclusions
In this series, the use of BioGlue in thoracic aortic surgery was associated with a very low incidence of anastomotic pseudoaneurysm formation in patients who survived the operation. Its use need not be discouraged on this basis for patients with acute aortic dissection or extreme tissue fragility.
Acknowledgements
We sincerely thank Dr. Joshua I. Weiner and Patricia LaFond, RN for their help in data collection.
Footnote
Conflicts of Interest: The abstract was presented at the American Association for Thoracic Surgery Aortic Symposium, New York, New York, May 12-13, 2016.
Ethical Statement: The Human Investigation Committee of Yale University approved this retrospective study (HIC # 1509016418).
References
- LeMaire SA, Green SY, Sharma K, et al. Aortic root replacement with stentless porcine xenografts: early and late outcomes in 132 patients. Ann Thorac Surg 2009;87:503-12; discussion 12-3. [Crossref] [PubMed]
- Guilmet D, Bachet J, Goudot B, et al. Use of biological glue in acute aortic dissection. Preliminary clinical results with a new surgical technique. J Thorac Cardiovasc Surg 1979;77:516-21. [PubMed]
- Glickman M, Gheissari A, Money S, et al. A polymeric sealant inhibits anastomotic suture hole bleeding more rapidly than gelfoam/thrombin: results of a randomized controlled trial. Arch Surg 2002;137:326-31; discussion 32. [Crossref] [PubMed]
- Köveker G, de Vivie ER, Hellberg KD. Clinical experience with fibrin glue in cardiac surgery. Thorac Cardiovasc Surg 1981;29:287-9. [Crossref] [PubMed]
- Bracey A, Shander A, Aronson S, et al. The Use of Topical Hemostatic Agents in Cardiothoracic Surgery. Ann Thorac Surg 2017;104:353-60. [Crossref] [PubMed]
- CryoLife. BioGlue surgical adhesive: Instrurction for use. 2014. Accessed March 18, 2016. Available online: http://www.cryolife.com/wp-content/uploads/stories/assets/docs/BG_Surgical_Adhesive_Syringe_IFU_dom.pdf
- Coselli JS, Bavaria JE, Fehrenbacher J, et al. Prospective randomized study of a protein-based tissue adhesive used as a hemostatic and structural adjunct in cardiac and vascular anastomotic repair procedures. J Am Coll Surg 2003;197:243-52; discussion 52-3. [Crossref] [PubMed]
- Passage J, Jalali H, Tam RK, et al. BioGlue Surgical Adhesive--an appraisal of its indications in cardiac surgery. Ann Thorac Surg 2002;74:432-7. [Crossref] [PubMed]
- Fehrenbacher JW, Siderys H. Use of BioGlue in aortic surgery: proper application techniques and results in 92 patients. Heart Surg Forum 2006;9:E794-9. [Crossref] [PubMed]
- Rylski B, Bavaria JE, Milewski RK, et al. Long-term results of neomedia sinus valsalva repair in 489 patients with type A aortic dissection. Ann Thorac Surg 2014;98:582-8; discussion 8-9. [Crossref] [PubMed]
- Raanani E, Latter DA, Errett LE, et al. Use of "BioGlue" in aortic surgical repair. Ann Thorac Surg 2001;72:638-40. [Crossref] [PubMed]
- Fink D, Klein JJ, Kang H, et al. Application of biological glue in repair of intracardiac structural defects. Ann Thorac Surg 2004;77:506-11. [Crossref] [PubMed]
- Luk A, David TE, Butany J. Complications of Bioglue postsurgery for aortic dissections and aortic valve replacement. J Clin Pathol 2012;65:1008-12. [Crossref] [PubMed]
- Weiner J, Widman S, Golek Z, et al. Role of bovine serum albumin-glutaraldehyde glue in the formation of anastomatic pseudoaneurysms. J Card Surg 2011;26:76-81. [Crossref] [PubMed]
- Alameddine A, Alimov VK, Rousou JA, et al. Aorto-pulmonary artery disruption following acute type-A aortic dissection repair with the use of BioGlue®. J Card Surg 2012;27:371-3. [Crossref] [PubMed]
- Rubio Alvarez J, Sierra Quiroga J, Martinez de Alegria A, et al. Pulmonary embolism due to biological glue after repair of type A aortic dissection. Interact Cardiovasc Thorac Surg 2011;12:650-1. [Crossref] [PubMed]
- Elefteriades JA. How I do it: utilization of high-pressure sealants in aortic reconstruction J Cardiothorac Surg 2009;4:27. [Crossref] [PubMed]
- Kazui T, Washiyama N, Bashar AH, et al. Role of biologic glue repair of proximal aortic dissection in the development of early and midterm redissection of the aortic root. Ann Thorac Surg 2001;72:509-14. [Crossref] [PubMed]
- Bavaria JE, Pochettino A, Brinster DR, et al. Prospective randomized study of Bioglue tissue adhesive during repair of acute type A aortic dissection. 81st Annual Meeting of the American Association for Thoracic Surgery; May 7, 2001; San Diego, CA2001, USA.
- Kazui T. What are the risks of using biologic glues Ann Thorac Surg 2003;75:1063-4. Reply. [Crossref] [PubMed]
- Downing SW. What are the risks of using biologic glues? Ann Thorac Surg 2003;75:1063-author reply 1063-4. [Crossref] [PubMed]
- Nishida H, Tabata M, Fukui T, et al. Surgical strategy and outcome for aortic root in patients undergoing repair of acute type A aortic dissection. Ann Thorac Surg 2016;101:1464-9. [Crossref] [PubMed]
- Ngaage DL, Edwards WD, Bell MR, et al. A cautionary note regarding long-term sequelae of biologic glue. J Thorac Cardiovasc Surg 2005;129:937-8. [Crossref] [PubMed]
- Fürst W, Banerjee A. Release of glutaraldehyde from an albumin-glutaraldehyde tissue adhesive causes significant in vitro and in vivo toxicity. Ann Thorac Surg 2005;79:1522-8; discussion 9. [Crossref] [PubMed]
- Hewitt CW, Marra SW, Kann BR, et al. BioGlue surgical adhesive for thoracic aortic repair during coagulopathy: efficacy and histopathology. Ann Thorac Surg 2001;71:1609-12. [Crossref] [PubMed]
- Stylli SS, Kumar A, Gonzales M, et al. The biocompatibility of BioGlue with the cerebral cortex: a pilot study. J Clin Neurosci 2004;11:631-5. [Crossref] [PubMed]
- Milano AD, Pratali S, Mecozzi G, et al. Fate of coronary ostial anastomoses after the modified Bentall procedure. Ann Thorac Surg 2003;75:1797-801; discussion 802.
- Ozaslan F, Wittlinger T, Monsefi N, et al. Long-term follow-up of supra-annular pulmonary autograft aortic root replacement in patients with bicuspid aortic valve. Eur J Cardiothorac Surg 2008;34:583-8; discussion 8. [Crossref] [PubMed]
- Westaby S, Saito S, Katsumata T. Acute type A dissection: conservative methods provide consistently low mortality. Ann Thorac Surg 2002;73:707-13. [Crossref] [PubMed]
- Bavaria JE, Brinster DR, Gorman RC, et al. Advances in the treatment of acute type A dissection: an integrated approach. Ann Thorac Surg 2002;74:S1848-52; discussion S57-63.


