Diagnosis and management of malignant pleural effusions: state of the art in 2017
Introduction
Malignant pleural effusion (MPE) is a commonly encountered complication of advanced malignancy. The incidence of pleural effusion is estimated to be greater than 150,000 cases of which lung cancer, breast cancer, and lymphoma are the most common causes but most malignancies have been reported to cause MPEs (1). Median survival following diagnosis ranges from 3 to 12 months and is dependent on the type of underlying malignancy, tumor characteristics, the extent of disease, comorbidities and the composition of pleural effusion (2-6). Despite this limited prognosis, predicting individual remaining life span is difficult which makes the goals of palliation and improving quality of life even more challenging. Burrows et al. found that only poor performance status correlated with mortality, lower Karnofsky scores predicted shorter survival (median survival of 1.1 months with a score <30 and 13.2 months with a score of >70) (3). A more recent study used performance status [Eastern Cooperative Oncology Group score (ECOG)] combined with other pleural fluid findings [lactate dehydrogenase (LDH) levels, neutrophil to lymphocyte ratio and tumor type], in determining a LENT score, which was found to be a better predictor of survival compared to performance status (ECOG) alone (6).
Diagnostic tools
Clinical presentation
The presentation of MPE can vary from no symptoms to acute respiratory distress. Dyspnea is the most common presenting symptom stemming predominantly from alteration of chest wall/diaphragmatic mechanics (1). Thus, the volume of pleural effusion may not necessarily correlate with the severity of their symptoms and difficult to predict the physiological sequelae, other factors such as other pulmonary/cardiac comorbidities may also be relevant (7). Additional symptoms related to MPE are chest pain which is more commonly seen in mesothelioma and is often dull rather than pleuritic (1). Most patients with MPEs have significant effusion and often chest exam can be abnormal (1).
Imaging techniques
Chest radiography
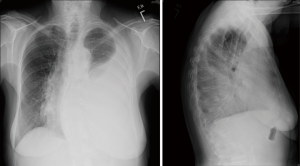
Plain chest radiograph features of MPE are characteristic (Figure 1), and chest X-ray is abnormal in the presence of 200 mL of pleural fluid on PA view and 50 mL on the lateral view. Most patients with MPE present with shortness of breath on exertion and their chest X-ray often shows moderate to large pleural effusions (80%), and 10% will have massive pleural effusion and 10% less than 500 mL (1,8-10).
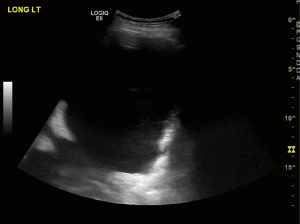
Ultrasound of the chest
Ultrasonography has a higher sensitivity in detection of pleural effusion than chest radiography as a screening tool (Figure 2) (11,12). It helps with the assessment of the thickness of the lining of the pleura and identifies pleural metastases. Pleural metastasis typically appears as relatively small hypo echoic lenticular masses having obtuse margins with the chest wall or large masses with a complex echogenicity (13). There is data supporting the use immediate pre-procedural ultrasonography to identify appropriate site for drainage, septations decreases the rates of complications and has become the standard of care (14-19). Ultrasound is a useful post-procedural tool for assessment of lung re-expansion post drainage and in suspected cases rapid identification of possible pneumothorax.
Computed tomography (CT)
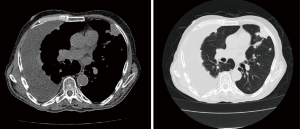
Contrast-enhanced CT scan of the chest may help differentiate between benign and malignant pleural disease (Figure 3). Pleural thickening and nodular lesions suggest the presence of malignant disease. Porcel et al. evaluated CT scan scoring system which included the presence of pleural lesion >1 cm, liver metastases, lung mass or lung nodule more than 1 cm, the absence of loculations, pericardial effusion and non-enlarged cardiac silhouette. CT score of ≥7 was found to predict malignancy with an 88% sensitivity and 94% specificity (20) Additional findings which are worrisome are medial pleural and interlobar fissure pleural thickening and nodularity (21).
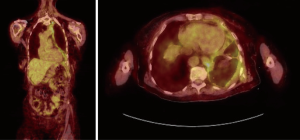
PET scan
Fluorodeoxyglucose (FDG) PET imaging is commonly used as part of staging evaluation for malignancies, however its value in predicting benign vs. malignant disease is limited due to the high false positive rate in patients with pleural infections and inflammation (22). Porcel et al. performed a meta-analysis of the accuracy of PET imaging for differentiating benign from MPEs. PET-CT had a sensitivity of 81% and specificity of 74% (23). PET imaging due to the high false positives and low specificity does not have a routine role in differentiating malignant versus benign pleural effusion (22,23) (Figure 4). Ultimately, the PET scan findings may be helpful in targeting certain anatomical areas of the pleura to biopsy, in cases of mixed disease such as mesothelioma and pleural asbestosis, this information may be invaluable (21).
Diagnostic pleural procedures
Thoracentesis
Thoracentesis is frequently performed for diagnosis and therapeutic reasons. Ultrasound examination is performed immediately before the procedure for direct guidance and appropriate entry point in identified and marked. Pleural fluid aspiration is carried out using aseptic precautions. There are no absolute contraindications for thoracentesis. There is no increased risk of bleeding due to mild to moderate coagulopathy and thrombocytopenia. Recent studies have also shown no increase in the risk of bleeding with uncorrected coagulopathy and other bleeding risks such as clopidogrel use, renal disease and thrombocytopenia (24,25). Swiderek et al. found that 60 mL pleural fluid is adequate for diagnosis of MPE. However, when thoracentesis is both therapeutic and diagnostic larger than 60 mL volume should be sent (22,26). MPE is usually an exudate, but 5–10% are transudates (9). Pleural fluid cytology is the simplest definitive way to diagnose MPE and can depend on tumor burden and type of tumor. The diagnostic rate of pleural fluid cytology is higher in adenocarcinoma and lower in mesothelioma (27-29). The mean sensitivity of thoracentesis in the diagnosis of malignancy by pleural fluid cytology is approximately 60% (22,30,31). Additional second specimen increases the yield by 27%, but more than >2 does not increase the diagnostic yield (31).
Blind closed pleural biopsy
The diagnostic yield of pleural fluid cytology is low, and when pleural fluid cytology is non-diagnostic in a patient with suspected MPE, a pleural biopsy is recommended.
A blind closed pleural biopsy is performed using an Abrams or Cope needle. Blind closed pleural biopsies have lower sensitivity due to the lower early stage and distribution of tumor (1). Despite the limitations practitioners around the world continue to perform the procedure as it requires limited experience, and the cost of equipment, team, and resources is lower than medical thoracoscopy (MT) (32). Few studies have shown slightly higher sensitivities and when combined with pleural fluid cytology can improve diagnostic sensitivity an additional 7–27% (22,30,33). The complication rates are as high as 14.4% with 9.4% incidence of pneumothorax (34).
Image-guided biopsy
CT-guided and ultrasound-guided biopsy can be performed to obtain pleural tissue for diagnosis. CT guided biopsy has a reported sensitivity of 76–88% and specificity of up to 100% for the diagnosis of malignant pleural thickening including a sensitivity of 83–86% for the diagnosis of mesothelioma which is superior to prior reports of blind closed pleural biopsy (35-38). US guided biopsies are also superior to blind closed pleural biopsy (14,39,40). Reported sensitivity range from 70% to 94% (35-37,39). In patients with suspected mesothelioma where there is no pleural effusion image-guided biopsy (CT or US guided) is preferred compared to closed pleural biopsy (41).
MT and video-assisted thoracoscopic surgery (VATS)
MT (also known as pleuroscopy) was popularized in 1910 by a Swedish internist, Hans Christian Jacobaeus (42). Originally through a cystoscope, the foundations for a better appreciation of pleural disease were initiated and developed into a diagnostic and therapeutic procedure. The evolution of MT into VATS has allowed for an even greater range of therapeutic solutions. MT differs in some ways from VATS in that it is performed in an endoscopy or operating room, with local anesthesia and/or moderate sedation, single port, and by a pulmonologist or surgeon. Both methods allow for direct visualization and biopsy of suspicious pleural abnormalities such as nodularity (Figure 5), masses, and thickening. Due to lower than accepted diagnostic rates for blind closed pleural biopsy which may be in combination or after a nondiagnostic pleural fluid cytology, a visualized directed pleural biopsy via MT or VATS may be needed. In a review of multiple case series, MT had a sensitivity of 92.6% (95% CI, 91.1–94%) for the diagnosis of malignant disease (43). In a randomized study (124 subjects) comparing image-guided biopsy (CT Abrams needle) vs. MT, the sensitivity for MT was higher (94.1% vs. 87.5%) but not statistically different (P=0.252) for malignancy. The use of ultrasound before MT has now become routine following several studies showing better visualization of the pleural space, which can reduce total procedure time, and prevent access failure (44,45)
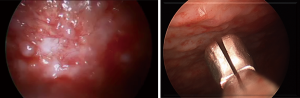
MT with local anesthetic has a low rate of complication and mortality despite the invasiveness of the procedure. Mortality rate related to medical thoracoscopy alone is approximately 0.34% as reported by Rahman et al. and may be linked to complications associated with talc (43). Major complications including empyema, hemorrhage, port site tumor growth (mesothelioma), bronchopleural fistula, postoperative pneumothorax or air leak and pneumonia were reported in 1.8% of cases (46).
VATS and MT is an invaluable diagnostic tool for the diagnosis of pleural mesothelioma especially considering the even lower diagnostic rate of pleural fluid cytology alone (26–32%) (22,47). Boutin et al. published their experience on 188 patients with pleural mesothelioma, 98% of whom were diagnosed on thoracoscopy (48). A recommendation from this study is to strongly consider port side radiation post procedure in patients with mesothelioma to decrease the risk of tract tumor seeding with malignant cells (48). Recent studies have shown no benefit of routine use of prophylactic radiotherapy following pleural interventions in patients with malignant mesothelioma (49,50).
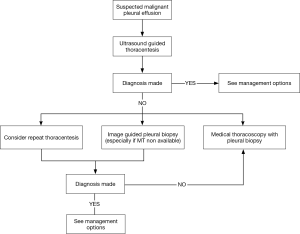
Figure 6 shows a modified diagnostic algorithm for patients with suspected MPE.
Management MPE
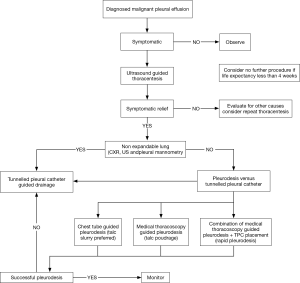
Once the diagnosis of MPE is made palliation of symptoms and improving the quality of life becomes the priority. Treatment approach varies depending on performance status, type of tumor and expected survival. In a patient who is asymptomatic and limited survival, an approach of best supportive care without any intervention is reasonable. Figure 7 shows a modified algorithm summarizing recent evidence and our approach.
Therapeutic thoracentesis
Therapeutic thoracentesis is typically the first step in management and should be performed in most patients with dyspnea (1,2). It allows for the assessment of improvement in clinical symptoms, re-expansion of the lung, and the rate of recurrence. Thoracentesis is a safe procedure, and the complication rates have decreased significantly with the routine use of pre-procedural ultrasound. The amount of pleural fluid that can be removed safely has been debated and some guidelines propose limiting drainage to 1–1.5 L. There is a concern for re-expansion pulmonary edema when large volume thoracentesis is performed and if pleural pressure can be kept above −20 cmH2O fluid removal can be continued. In a large series of patients undergoing large volume thoracentesis the incidence of clinical reexpansion pulmonary edema is rare and in our opinion, the amount of drainage should not be limited to 1–1.5 L (51). In another study development of chest discomfort was associated with an unsafe drop in pleural pressures and when pleural manometry is not available symptoms can be a valuable surrogate (52). Recently, a commercially available device is available for measuring pleural manometry digitally without the need of U-tube manometer. Pleural manometry, chest imaging and ultrasound of the chest are useful in assessing re-expansion post pleural drainage and can help in deciding the intervention for palliation in case of recurrence of symptomatic pleural effusion (53).
Pleurodesis
Pleurodesis is the fusion of the parietal and visceral pleura, which leads to obliteration of the pleural space and prevents accumulation of pleural effusion. The exact mechanism of pleurodesis is unclear but it is suspected to be due to inflammation/fibrosis via transforming growth factor beta (54). Mechanical and chemical intrapleural instillation of various agents such as talc, bleomycin, tetracycline, corynebacterium parvum and doxycycline has been used to achieve pleurodesis (55). Walker-Renard et al. reviewed studies on patients with recurrent symptomatic pleural effusion. A total of 1,168 patients with MPE were analyzed for efficacy. Complete response occurred in 64% patients who underwent chemical pleurodesis. The success rate of the pleurodesis agents varied from 0% with etoposide to 93% with talc. The most commonly adverse effects were pain (23%) and fever (19%) (56). Efficacy of patient rotation after instillation of intrapleural sclerosants has been evaluated in randomized trials there was no difference in distribution of sclerosants and pleurodesis rates. Patient rotation is not necessary after instillation of intrapleural sclerosants (57,58).
In a Cochrane meta-analysis of 1,499 subjects, the use of sclerosants correlated with an increased efficacy of pleurodesis (RR of non-recurrence 1.20, 95% CI, 1.04–1.38) favoring the use of sclerosants. Compared to different sclerosants, talc was found to be the most efficacious (RR of non-recurrence 1.34, 95% CI, 1.16–1.55) with no increased mortality post pleurodesis (55). Talc appears to be the most effective and least expensive agent (59-65), however, there is increasing support for povidone-iodine as an equally efficacious agent, which may also be safer and more cost effective (depending on country of use). Talc is a trilayered magnesium silicate sheet, asbestos free, and is generally <50 µm. It is sterilized prior by dry heat, ethylene oxide or γ irradiation and remains culture negative for at least 1 year (1,2).
Talc slurry versus poudrage
Talc can be administered at thoracoscopy via an atomizer (talc poudrage) or in a suspension form (talc slurry) via a chest tube. The 2004 Cochrane review of pleurodesis suggests talc was the most effective sclerosant and also found talc poudrage at thoracoscopy to have an improved relative risk of non-recurrence over talc slurry (55).
Dresler et al. in a large randomized trial compared thoracoscopy guided talc poudrage (TTI) to talc slurry (TS) via a chest tube. There was no statistical difference between the two interventions in successful 30-day outcomes (TTI, 78%; TS, 71%). A subgroup analysis of patients with primary lung or breast cancer had higher success with TTI than with TS (82% vs. 67%, P=0.04). Common morbidity included fever (68%), dyspnea (16%), and pain (5–10%).Respiratory complications were more common following TTI than TS (14% vs. 6%). Respiratory failure was observed in 4% of TS patients and 8% of TTI patients, accounting for 5 toxic deaths and 6 toxic deaths, respectively (46). There is conflicting evidence regarding the superiority of talc poudrage over talc slurry and to answer the question currently a large multicenter randomized trial (TAPPS trial) is comparing talc poudrage via medical thoracoscopy to talc slurry via chest tube (12–14 F Seldinger technique) with a primary end point of pleurodesis failure at 3 months (66).
TIME-1 study, a multicenter randomized study, compared patients with large versus small-bore chest tube and the impact of non-steroidal anti-inflammatory drugs (NSAIDs) on the effectiveness of talc pleurodesis. Pain scores in the opiate group vs. the NSAID group were not significantly different, but the NSAID group required more rescue analgesia. There was no difference in the rates of pleurodesis failure between the opiate group and the NSAID group. Pain scores were lower in the 12-F chest tube group. The 12-F vs. 24-F chest tubes were associated with higher pleurodesis failure (30% vs. 24%). This is an important finding and argues for larger-bore chest tubes for pleurodesis in MPE (67,68).
Tunneled pleural catheter (TPC)
The use of TPC is increasingly common for the management of MPE TPC is a silicone tube that is placed into the pleural cavity, tunneled subcutaneously with a small cuff, and the other end exiting the patient with a one-way valve. This allows easy drainage at home or in an ambulatory setting, by patients and/or their caregivers. There is increasing evidence that TPC are safe and effective in managing patient symptoms and improving QoL. A systematic review of 19 studies with a total of 1,370 patients showed symptomatic improvement was reported in 95.6%. Spontaneous pleurodesis occurred in 45.6% (69). It remains unclear if removal of the catheter is from actual pleurodesis (fusion of visceral/parietal pleura) vs. cessation of pleural effusion accumulation. While pleurodesis is only an option for patients with good or significant lung re-expansion, TPC can be used for patients with trapped lung as well with good symptom control.
Putnam et al. in a randomized study comparing TPC to doxycycline pleurodesis showing no difference in the degree of symptomatic improvement in dyspnea and quality of life with either interventions (70). TIME-2, an unblinded randomized controlled trial, compared TPC and talc slurry via chest tube for pleurodesis. Dyspnea improved in both groups, and there was no significant difference in the first 42 days. However, there was a statistically significant improvement in dyspnea in the TPC group at 6 months. The length of initial hospitalization was significantly shorter in the TPC group with a median of 0 and 4 days for the talc group (71). Several large series have shown similar benefit in symptom control and improvement in QoL with low complication rates (72-76).
TPC related pleurodesis has been reported to occur between 29 to 59 days post placement (72,77,78). Van Meter et al. in a systematic review reported an overall spontaneous pleurodesis rate of 45% (69); Warren et al. reported a spontaneous pleurodesis rate of 58% (77). Tremblay et al. reported when including patients who may be suitable for pleurodesis, spontaneous pleurodesis rates increase to 70% (79). In a recent randomized trial, Impact of Aggressive versus Standard Drainage Regimen Using a Long-Term Indwelling Pleural Catheter trial (ASAP trial), rates of spontaneous pleurodesis were higher in patients daily drainage compared to the every other day drainage of pleural fluid via a TPC (47% vs. 24%, respectively; P = 0.003). Median time to spontaneous pleurodesis was shorter in the daily drainage group (54 days) as compared with the every other day drainage (90 days) (80).
The incidence TPC related infections are low range from 0% to 12% (81). A large international multicenter review characterized 1,021 patients with TPC found an infection rate of only 4.8% (82). Pleurodesis is common after TPC-related pleural infection and, in one study, allowed removal of the catheter in 62% of patients (80% in those with S. aureus empyema) (82). TPC guided talc pleurodesis is another option and is being currently studied in a large multicenter randomized trial (IPC plus trial) (83).
Rapid pleurodesis
One of the major drawbacks of pleurodesis is that it often necessitates a 5- to 7-day hospitalization (70,84). TPCs alone have been found to cause spontaneous pleurodesis and in a randomized multicenter study with aggressive daily drainage, it was 54 days compared to a less aggressive interval draining (90 days) (55,72). There is an inherent infectious risk with TPCs as well as the need for assistance with home drainage (69,82,85).
A rapid pleurodesis procedure, using the combination of thoracoscopy guided talc delivery for pleurodesis with TPC insertion at the same procedure (Figure 8) takes advantages of both management strategies and minimizes some disadvantages. This method has previously been shown in two series to decrease hospital length of stay (mean 1.8–2 days), and duration of TPC use (mean 8–10 days) measured by time to pleurodesis while significantly improving dyspnea and quality of life in patients with MPE (86,87). However, from a cost utilization, this method may be the least cost-effective option (88). Overall pleurodesis rates in patients who underwent rapid pleurodesis with the combination method were 92% (86,87).

Conclusions
MPE is a commonly encountered clinical problem where QoL and palliation are the paramount goal in its management. Our understanding of best practices for diagnosis and therapeutic options are continuing to expand with ongoing trials to further refine our management.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- American Thoracic S. Management of malignant pleural effusions. Am J Respir Crit Care Med 2000;162:1987-2001. [Crossref] [PubMed]
- Roberts ME, Neville E, Berrisford RG, et al. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii32-40. [Crossref] [PubMed]
- Burrows CM, Mathews WC, Colt HG. Predicting survival in patients with recurrent symptomatic malignant pleural effusions: an assessment of the prognostic values of physiologic, morphologic, and quality of life measures of extent of disease. Chest 2000;117:73-8. [Crossref] [PubMed]
- Bielsa S, Salud A, Martinez M, et al. Prognostic significance of pleural fluid data in patients with malignant effusion. Eur J Intern Med 2008;19:334-9. [Crossref] [PubMed]
- Pilling JE, Dusmet ME, Ladas G, et al. Prognostic factors for survival after surgical palliation of malignant pleural effusion. J Thorac Oncol 2010;5:1544-50. [Crossref] [PubMed]
- Clive AO, Kahan BC, Hooper CE, et al. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax 2014;69:1098-104. [Crossref] [PubMed]
- Doelken P, Abreu R, Sahn SA, et al. Effect of thoracentesis on respiratory mechanics and gas exchange in the patient receiving mechanical ventilation. Chest 2006;130:1354-61. [Crossref] [PubMed]
- Chernow B, Sahn SA. Carcinomatous involvement of the pleura: an analysis of 96 patients. Am J Med 1977;63:695-702. [Crossref] [PubMed]
- Sahn SA. State of the art. The pleura. Am Rev Respir Dis 1988;138:184-234. [Crossref] [PubMed]
- Maher GG, Berger HW. Massive pleural effusion: malignant and nonmalignant causes in 46 patients. Am Rev Respir Dis 1972;105:458-60. [PubMed]
- Yousefifard M, Baikpour M, Ghelichkhani P, et al. Screening Performance Characteristic of Ultrasonography and Radiography in Detection of Pleural Effusion; a Meta-Analysis. Emerg (Tehran) 2016;4:1-10. [PubMed]
- Eibenberger KL, Dock WI, Ammann ME, et al. Quantification of pleural effusions: sonography versus radiography. Radiology 1994;191:681-4. [Crossref] [PubMed]
- Wernecke K. Ultrasound study of the pleura. Eur Radiol 2000;10:1515-23. [Crossref] [PubMed]
- Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest 2003;123:436-41. [Crossref] [PubMed]
- Raptopoulos V, Davis LM, Lee G, et al. Factors affecting the development of pneumothorax associated with thoracentesis. AJR Am J Roentgenol 1991;156:917-20. [Crossref] [PubMed]
- Gordon CE, Feller-Kopman D, Balk EM, et al. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010;170:332-9. [Crossref] [PubMed]
- Barnes TW, Morgenthaler TI, Olson EJ, et al. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound 2005;33:442-6. [Crossref] [PubMed]
- Sachdeva A, Shepherd RW, Lee HJ. Thoracentesis and thoracic ultrasound: state of the art in 2013. Clin Chest Med 2013;34:1-9. [Crossref] [PubMed]
- Cantey EP, Walter JM, Corbridge T, et al. Complications of thoracentesis: incidence, risk factors, and strategies for prevention. Curr Opin Pulm Med 2016;22:378-85. [Crossref] [PubMed]
- Porcel JM, Pardina M, Bielsa S, et al. Derivation and validation of a CT scan scoring system for discriminating malignant from benign pleural effusions. Chest 2015;147:513-9. [Crossref] [PubMed]
- Wang ZJ, Reddy GP, Gotway MB, et al. Malignant pleural mesothelioma: evaluation with CT, MR imaging, and PET. Radiographics 2004;24:105-19. [Crossref] [PubMed]
- Hooper C, Lee YC, Maskell N, et al. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii4-17. [Crossref] [PubMed]
- Porcel JM, Hernandez P, Martinez-Alonso M, et al. Accuracy of fluorodeoxyglucose-PET imaging for differentiating benign from malignant pleural effusions: a meta-analysis. Chest 2015;147:502-12. [Crossref] [PubMed]
- Puchalski JT, Argento AC, Murphy TE, et al. The safety of thoracentesis in patients with uncorrected bleeding risk. Ann Am Thorac Soc 2013;10:336-41. [Crossref] [PubMed]
- Zalt MB, Bechara RI, Parks C, et al. Effect of routine clopidogrel use on bleeding complications after ultrasound-guided thoracentesis. J Bronchology Interv Pulmonol 2012;19:284-7. [Crossref] [PubMed]
- Swiderek J, Morcos S, Donthireddy V, et al. Prospective study to determine the volume of pleural fluid required to diagnose malignancy. Chest 2010;137:68-73. [Crossref] [PubMed]
- Johnston WW. The malignant pleural effusion. A review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer 1985;56:905-9. [Crossref] [PubMed]
- Hsu C. Cytologic detection of malignancy in pleural effusion: a review of 5,255 samples from 3,811 patients. Diagn Cytopathol 1987;3:8-12. [Crossref] [PubMed]
- Starr RL, Sherman ME. The value of multiple preparations in the diagnosis of malignant pleural effusions. A cost-benefit analysis. Acta Cytol 1991;35:533-7. [PubMed]
- Prakash UB, Reiman HM. Comparison of needle biopsy with cytologic analysis for the evaluation of pleural effusion: analysis of 414 cases. Mayo Clin Proc 1985;60:158-64. [Crossref] [PubMed]
- Garcia LW, Ducatman BS, Wang HH. The value of multiple fluid specimens in the cytological diagnosis of malignancy. Mod Pathol 1994;7:665-8. [PubMed]
- Baumann MH. Closed pleural biopsy: not dead yet! Chest 2006;129:1398-400. [Crossref] [PubMed]
- Nance KV, Shermer RW, Askin FB. Diagnostic efficacy of pleural biopsy as compared with that of pleural fluid examination. Mod Pathol 1991;4:320-4. [PubMed]
- Pereyra MF, San-Jose E, Ferreiro L, et al. Role of blind closed pleural biopsy in the managment of pleural exudates. Can Respir J 2013;20:362-6. [Crossref] [PubMed]
- Metintaş M, Ozdemir N, Isiksoy S, et al. CT-guided pleural needle biopsy in the diagnosis of malignant mesothelioma. J Comput Assist Tomogr 1995;19:370-4. [Crossref] [PubMed]
- Adams RF, Gray W, Davies RJ, et al. Percutaneous image-guided cutting needle biopsy of the pleura in the diagnosis of malignant mesothelioma. Chest 2001;120:1798-802. [Crossref] [PubMed]
- Benamore RE, Scott K, Richards CJ, et al. Image-guided pleural biopsy: diagnostic yield and complications. Clin Radiol 2006;61:700-5. [Crossref] [PubMed]
- Adams RF, Gleeson FV. Percutaneous image-guided cutting-needle biopsy of the pleura in the presence of a suspected malignant effusion. Radiology 2001;219:510-4. [Crossref] [PubMed]
- Maskell NA, Gleeson FV, Davies RJ. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet 2003;361:1326-30. [Crossref] [PubMed]
- Heilo A, Stenwig AE, Solheim OP. Malignant pleural mesothelioma: US-guided histologic core-needle biopsy. Radiology 1999;211:657-9. [Crossref] [PubMed]
- Stigt JA, Boers JE, Groen HJ. Analysis of "dry" mesothelioma with ultrasound guided biopsies. Lung Cancer 2012;78:229-33. [Crossref] [PubMed]
- Shojaee S, Lee HJ. Thoracoscopy: medical versus surgical-in the management of pleural diseases. J Thorac Dis 2015;7:S339-51. [PubMed]
- Rahman NM, Ali NJ, Brown G, et al. Local anaesthetic thoracoscopy: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii54-60. [Crossref] [PubMed]
- Macha HN, Reichle G, von Zwehl D, et al. The role of ultrasound assisted thoracoscopy in the diagnosis of pleural disease. Clinical experience in 687 cases. Eur J Cardiothorac Surg 1993;7:19-22. [Crossref] [PubMed]
- Medford AR, Agrawal S, Bennett JA, et al. Thoracic ultrasound prior to medical thoracoscopy improves pleural access and predicts fibrous septation. Respirology 2010;15:804-8. [Crossref] [PubMed]
- Dresler CM, Olak J, Herndon JE 2nd, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest 2005;127:909-15. [Crossref] [PubMed]
- Renshaw AA, Dean BR, Antman KH, et al. The role of cytologic evaluation of pleural fluid in the diagnosis of malignant mesothelioma. Chest 1997;111:106-9. [Crossref] [PubMed]
- Boutin C, Rey F, Viallat JR. Prevention of malignant seeding after invasive diagnostic procedures in patients with pleural mesothelioma. A randomized trial of local radiotherapy. Chest 1995;108:754-8. [Crossref] [PubMed]
- Clive AO, Taylor H, Dobson L, et al. Prophylactic radiotherapy for the prevention of procedure-tract metastases after surgical and large-bore pleural procedures in malignant pleural mesothelioma (SMART): a multicentre, open-label, phase 3, randomised controlled trial. Lancet Oncol 2016;17:1094-104. [Crossref] [PubMed]
- O'Rourke N, Garcia JC, Paul J, et al. A randomised controlled trial of intervention site radiotherapy in malignant pleural mesothelioma. Radiother Oncol 2007;84:18-22. [Crossref] [PubMed]
- Feller-Kopman D, Berkowitz D, Boiselle P, et al. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg 2007;84:1656-61. [Crossref] [PubMed]
- Feller-Kopman D, Walkey A, Berkowitz D, et al. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest 2006;129:1556-60. [Crossref] [PubMed]
- Salamonsen MR, Lo AK, Ng AC, et al. Novel use of pleural ultrasound can identify malignant entrapped lung prior to effusion drainage. Chest 2014;146:1286-93. [Crossref] [PubMed]
- Shojaee S, Voelkel N, Farkas L, et al. Transforming growth factor-beta1 rise in pleural fluid after tunneled pleural catheter placement: pilot study. J Bronchology Interv Pulmonol 2013;20:304-8. [Crossref] [PubMed]
- Shaw P, Agarwal R. Pleurodesis for malignant pleural effusions. Cochrane Database Syst Rev 2004.CD002916. [PubMed]
- Walker-Renard PB, Vaughan LM, Sahn SA. Chemical pleurodesis for malignant pleural effusions. Ann Intern Med 1994;120:56-64. [Crossref] [PubMed]
- Mager HJ, Maesen B, Verzijlbergen F, et al. Distribution of talc suspension during treatment of malignant pleural effusion with talc pleurodesis. Lung Cancer 2002;36:77-81. [Crossref] [PubMed]
- Dryzer SR, Allen ML, Strange C, et al. A comparison of rotation and nonrotation in tetracycline pleurodesis. Chest 1993;104:1763-6. [Crossref] [PubMed]
- Zimmer PW, Hill M, Casey K, et al. Prospective randomized trial of talc slurry vs bleomycin in pleurodesis for symptomatic malignant pleural effusions. Chest 1997;112:430-4. [Crossref] [PubMed]
- Noppen M, Degreve J, Mignolet M, et al. A prospective, randomised study comparing the efficacy of talc slurry and bleomycin in the treatment of malignant pleural effusions. Acta Clin Belg 1997;52:258-62. [Crossref] [PubMed]
- Hamed H, Fentiman IS, Chaudary MA, et al. Comparison of intracavitary bleomycin and talc for control of pleural effusions secondary to carcinoma of the breast. Br J Surg 1989;76:1266-7. [Crossref] [PubMed]
- Fentiman IS, Rubens RD, Hayward JL. A comparison of intracavitary talc and tetracycline for the control of pleural effusions secondary to breast cancer. Eur J Cancer Clin Oncol 1986;22:1079-81. [Crossref] [PubMed]
- Diacon AH, Wyser C, Bolliger CT, et al. Prospective randomized comparison of thoracoscopic talc poudrage under local anesthesia versus bleomycin instillation for pleurodesis in malignant pleural effusions. Am J Respir Crit Care Med 2000;162:1445-9. [Crossref] [PubMed]
- Ong KC, Indumathi V, Raghuram J, et al. A comparative study of pleurodesis using talc slurry and bleomycin in the management of malignant pleural effusions. Respirology 2000;5:99-103. [Crossref] [PubMed]
- Kuzdzał J, Sladek K, Wasowski D, et al. Talc powder vs doxycycline in the control of malignant pleural effusion: a prospective, randomized trial. Med Sci Monit 2003;9:PI54-9. [PubMed]
- Bhatnagar R, Laskawiec-Szkonter M, Piotrowska HE, et al. Evaluating the efficacy of thoracoscopy and talc poudrage versus pleurodesis using talc slurry (TAPPS trial): protocol of an open-label randomised controlled trial. BMJ Open 2014;4:e007045. [Crossref] [PubMed]
- Rahman NM, Pepperell J, Rehal S, et al. Effect of Opioids vs NSAIDs and Larger vs Smaller Chest Tube Size on Pain Control and Pleurodesis Efficacy Among Patients With Malignant Pleural Effusion: The TIME1 Randomized Clinical Trial. JAMA 2015;314:2641-53. [Crossref] [PubMed]
- Hayek SM, Lawrence MM. Size Matters in Chest Tubes-Efficacy of Analgesia and Pleurodesis in Patients With Malignant Pleural Effusion. JAMA Oncol 2016;2:1643-4. [Crossref] [PubMed]
- Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011;26:70-6. [Crossref] [PubMed]
- Putnam JB Jr, Light RW, Rodriguez RM, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer 1999;86:1992-9. [Crossref] [PubMed]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012;307:2383-9. [Crossref] [PubMed]
- Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest 2006;129:362-8. [Crossref] [PubMed]
- Freeman RK, Ascioti AJ, Mahidhara RS. A propensity-matched comparison of pleurodesis or tunneled pleural catheter in patients undergoing diagnostic thoracoscopy for malignancy. Ann Thorac Surg 2013;96:259-63: discussion 63-4.
- Demmy TL, Gu L, Burkhalter JE, et al. Optimal management of malignant pleural effusions (results of CALGB 30102). J Natl Compr Canc Netw 2012;10:975-82. [Crossref] [PubMed]
- Hunt BM, Farivar AS, Vallieres E, et al. Thoracoscopic talc versus tunneled pleural catheters for palliation of malignant pleural effusions. Ann Thorac Surg 2012;94:1053-7; discussion 7-9. [Crossref] [PubMed]
- Srour N, Amjadi K, Forster A, et al. Management of malignant pleural effusions with indwelling pleural catheters or talc pleurodesis. Can Respir J 2013;20:106-10. [Crossref] [PubMed]
- Warren WH, Kalimi R, Khodadadian LM, et al. Management of malignant pleural effusions using the Pleur(x) catheter. Ann Thorac Surg 2008;85:1049-55. [Crossref] [PubMed]
- Suzuki K, Servais EL, Rizk NP, et al. Palliation and pleurodesis in malignant pleural effusion: the role for tunneled pleural catheters. J Thorac Oncol 2011;6:762-7. [Crossref] [PubMed]
- Tremblay A, Mason C, Michaud G. Use of tunnelled catheters for malignant pleural effusions in patients fit for pleurodesis. Eur Respir J 2007;30:759-62. [Crossref] [PubMed]
- Wahidi MM, Reddy C, Yarmus L, et al. Randomized Trial of Pleural Fluid Drainage Frequency in Patients with Malignant Pleural Effusions. The ASAP Trial. Am J Respir Crit Care Med 2017;195:1050-7. [Crossref] [PubMed]
- Lui MM, Thomas R, Lee YC. Complications of indwelling pleural catheter use and their management. BMJ Open Respir Res 2016;3:e000123. [Crossref] [PubMed]
- Fysh ET, Tremblay A, Feller-Kopman D, et al. Clinical outcomes of indwelling pleural catheter-related pleural infections: an international multicenter study. Chest 2013;144:1597-602. [Crossref] [PubMed]
- Bhatnagar R, Kahan BC, Morley AJ, et al. The efficacy of indwelling pleural catheter placement versus placement plus talc sclerosant in patients with malignant pleural effusions managed exclusively as outpatients (IPC-PLUS): study protocol for a randomised controlled trial. Trials 2015;16:48. [Crossref] [PubMed]
- Putnam JB Jr, Walsh GL, Swisher SG, et al. Outpatient management of malignant pleural effusion by a chronic indwelling pleural catheter. Ann Thorac Surg 2000;69:369-75. [Crossref] [PubMed]
- Musani AI, Haas AR, Seijo L, et al. Outpatient management of malignant pleural effusions with small-bore, tunneled pleural catheters. Respiration 2004;71:559-66. [Crossref] [PubMed]
- Reddy C, Ernst A, Lamb C, et al. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest 2011;139:1419-23. [Crossref] [PubMed]
- Krochmal R, Reddy C, Yarmus L, et al. Patient evaluation for rapid pleurodesis of malignant pleural effusions. J Thorac Dis 2016;8:2538-43. [Crossref] [PubMed]
- Shafiq M, Frick KD, Lee H, et al. Management of Malignant Pleural Effusion: A Cost-Utility Analysis. J Bronchology Interv Pulmonol 2015;22:215-25. [Crossref] [PubMed]