Donors after cardiocirculatory death and lung transplantation
Introduction
The number of patients actively awaiting lung transplantation (LTx) is more than the number of suitable donor lungs. The percentage of lung retrieval rate is lower when compared to other solid organs. Brain death itself leads to hemodynamic, metabolic and neuroendocrine abnormalities resulting in so-called neurogenic pulmonary edema (1,2). This initial insult in combination with possible airway aspiration, respiratory tract infection, atelectasis and pulmonary contusion, may all contribute to lung damage before harvest (1).
The use of lungs from donation after cardiocirculatory death (DCD) donors is one of the options to avoid organ shortage in LTx (3-16). The number of lung transplants performed from DCD donors is increasing. A recent International Society for Heart and Lung Transplantation (ISHLT) DCD Registry Report included 306 recipients among ten centers worldwide (12). Several centers published their experience, most of them with excellent or at least equal results compared to brain-dead donors (5,14,17-28).
The first successful attempt of human LTx (29), and the first long-term successful human LTx (30) utilized DCD donors. Thereafter the concept of brain death and organ donation after brain death (DBD) became more widely accepted (11) and because of this DCD was largely abandoned.
Proof of concept and experimental background
Thomas M. Egan reintroduced the concept of LTx from DCD donors in 1991 following a series of dog experiments (31). He showed that the lung may remain viable for a certain period after death as a result of the oxygen reserve present in the alveoli.
To investigate the hypothesis that lungs may be suitable for transplant even if explanted at substantial interval after death, Egan et al., used a canine single left lung transplant model (31). They retrieved left lungs at 1, 2, or 4 h after death from non-ventilated donors. Following the transplantation, they ligated the contralateral pulmonary artery and bronchus 1 h after transplantation to force the recipients survive solely on the transplanted lung retrieved from DCD donor. All recipients of lungs retrieved 1 h after death survived the 8-h observation period with good gas exchange. Two of the five recipients of 2-h cadaver lungs survived with good gas exchange, whereas gas exchange and survival were poor in recipients of lungs retrieved 4 h after death (31).
In order to find out the time course of pulmonary cell death after circulatory arrest D’Armini et al. from Egan’s group used trypan blue dye exclusion to quantitate lung cell death at postmortem intervals in rats. Postmortem mechanical ventilation with oxygen appeared to delay lung death in the rat DCD model (32).
To determine postmortem adenine nucleotide tissue levels in the lung and their relationship to lung viability D’Armini et al. showed that by 4 h after death, the viability was 85% in the O2-ventilated cadaver rat lungs, significantly higher than in the N2-ventilated (43%) and in the non-ventilated (48%) lungs (33).
In a dog model, Ulicny et al. retrieved lungs 4 h after death from ventilated DCD donors (34). Four of six recipients of oxygen-ventilated cadaver lungs survived 8 h with good gas exchange whereas two of six recipients of non-ventilated lungs survived with poor gas exchange. With additional canine studies, they demonstrated benefit of flushing lungs with solution containing a free radical scavenger, dimethylthiourea (35,36). Donor lung ventilation with alveolar gas (20% O2, 5% CO2, balanced N2) during 4-h warm ischemic time (WIT) did not result in improved lung function (37). DCD donors ventilated with 100% O2 prior to organ retrieval showed superior pulmonary function after transplantation compared with lungs grafts ventilated with alveolar gas (37).
Rega et al. showed that NAC administered before or shortly after death attenuated early ischemia-reperfusion injury via up-regulation of glutathione (38).
In a pig model, after 1 h in situ WIT the lungs were either topically cooled or ventilated for 3 h. Topically cooled lungs showed better function compared to ventilation-only group (39).
In a pig DCD model, donors with increasing time intervals of 1, 2, and 3 h and donors from heart-beating animals were assessed in ex vivo perfusion system. They found a strong correlation between the increase of IL-1beta concentration and the increase in pulmonary vascular resistance, mean airway pressure, and wet-to-dry weight ratio. They concluded that IL-1 beta in bronchial lavage fluid might be a useful, non-invasive marker that can predict the viability of the pulmonary graft from the DCD donors (40).
In dog model, Dougherty et al. were able to reduce the core temperature to 2 to 7 °C when one lung was ventilated with air delivered at subzero temperature (−10 to −15 °C) during 1 h (41). However, recipients did not survive on this lung alone because of the development capillary leak with edema as a result of the freezing damage (41). In a dog model Watanabe et al. were successful in transplanting DCD donor lungs that were cooled for 2 h by filling one hemithorax with cold air (42). Steen et al. in a pig DCD model with open chest, cooled donor lungs with saline slush placed in both pleural cavities (43). Lung core temperature decreased to less than 10 °C within 40 minutes and topical cooling was continued for 6 h. All six recipients survived for 24 h on the transplanted left lung with the exclusion of the right native lung (43). In order to create a clinically relevant situation, Steen’s group cooled the lungs topically in situ by continuous infusion of cold preservation solution via two intrapleural drains inserted via two small intercostal incisions (44).
The efficacy of partial liquid ventilation (PLV) with perfluorocarbon in lung protection during hypotension and cardiac arrest has been studied by Yoshida et al. (45). Using rabbit lungs, they maintained hypotension at <50 mmHg for 1 h followed by 2-h cardiac arrest. Histologic evaluation after perfusion of the preservation solution revealed that alveolar structure was damaged significantly less and cell infiltration was milder in the PLV groups than in the control group (45). Tissue IL-8 in the PLV groups remained at baseline concentrations during the study period. They concluded that PLV suppresses lung injury when compared with gas-controlled ventilation (45).
Okazaki et al. evaluated the optimal time for post-mortem heparinization in canine LTx from DCD donors (46). The cadaver donors were assigned randomly to one of five study groups. They reported that the optimal time for post-mortem heparinization in LTx from DCD donors was approximately 30 minutes after cardiac arrest (46).
Using ex vivo lung perfusion (EVLP) method we demonstrated that administration of urokinase during EVLP after 3 h of warm ischemia improved lung function by dissolving microthrombi with its fibrinolytic action (47).
We also investigated the impact of topical cooling solution and prediction of graft function from DCD donors (48). We found that topical cooling with Perfadex after 3 h of death resulted in improved graft function compared to saline group. However, graft parameters were comparable between saline and Perfadex groups after 1 h of warm ischemia (48).
To assess the surfactant alterations in DCD donor lungs (49) we showed that surfactant function decreases with increased WITs. This was proven by significantly different adsorption and surface tension in DCD groups compared with heart-beating donor (HBD) group (49).
In another study, we tested whether an injured lung graft from a category-3 DCD donor could be reconditioned with EVLP by intra-bronchial diluted surfactant lavage prior to transplantation (50). Our data demonstrated the feasibility of reconditioning and transplantation of an acutely damaged lung graft due to aspiration from a category-3 DCD donor (50).
Martens et al. demonstrated that warm ischemic injury in DCD donation could be attenuated by steroids when given prior to warm ischemia and during EVLP (51).
In a mice model, Huerter et al. demonstrated that adenosine A2B receptor (A2BR) antagonism attenuated lung ischemia reperfusion injury and augments reconditioning of DCD lungs by EVLP (52). The protective effects of A2BR antagonist (ATL802) might involve targeting A2BRs on alveolar epithelial cells to prevent IL-8 production. A2BR might be a novel therapeutic target for mitigating ischemia reperfusion injury to increase the success of LTx (52).
Clinical experience with DCD donors
Definition and categories
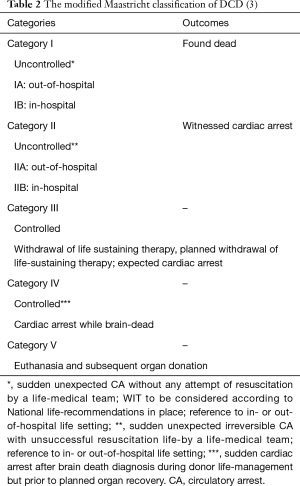
DCD donors are defined as when organs are removed from donors after cardiac arrest (1). According to Maastricht classification, there are four types of DCD donors (Table 1) (53). The first two categories are uncontrolled DCD (uDCD) donors. An uDCD donor may occur when a person dies unexpectedly. In these cases, the deceased person may become a potential donor if his or her organs can be adequately preserved inside the cadaver before organ retrieval and if the consent for the retrieval of organs can be obtained from the relatives (1). The exact length of the postmortem WIT is often not known. As organ function in these donors cannot be assessed before death, viability should be properly evaluated afterwards before organ transplantation to reduce the risk of primary non-function (1,6,8).
In the controlled DCD (cDCD) donors (categories III and IV), pulmonary graft assessment can be made after informed consent in the hours before withdrawal of life support in the same way as practiced in the HBD (chest X-ray, oxygenation, bronchoscopy) (1). The warm ischemic period of the graft is limited to 10 to 15 minutes after death certification if withdrawal of life support is executed in the operating room. Lungs can be inspected in situ, and preserved in the standard way (1). Recently, modified Maastricht classification of DCD has been published (Table 2) (3).
Definitions of WIT
The length of tolerable WIT for DCD donor lungs remains debatable; however, the majority of experimental data suggest that lungs remain viable for at least 60 to 90 min after circulatory arrest (1,2,54,55).
The clinical limit and most relevant definition of WIT for DCD donor lungs is still debatable (54). It has have been recommended to record prospectively post-withdrawal and postmortem DCD donor hemodynamics and oximetry in order to determine the range, pattern, and potential clinical relevance to DCD clinical lung transplant outcomes (54). Levvey et al. from Alfred Hospital, Melbourne recommended different definitions of WIT including the timing of withdrawal, systolic blood pressure (sBP) less than 50 mmHg, initiation of ventilation or the onset of pulmonary arterial flush (54). They suggested WIT definition starting when sBP <50 mmHg and finishing with cold arterial flush (54). This group emphasized the importance of prospectively collecting data on all potential DCD lung donors and to correlate these with clinical outcomes (54). Definitions that start with sBP <50 mmHg represent the start of serious hemodynamic compromise and might better correlate with clinically significant loss of organ perfusion (54).
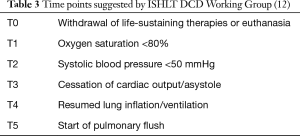
In order to standardize the definitions around important times in DCD donation process, ISHLT DCD Working Group recommended the following times points and intervals (12). Table 3 and Figure 1 show schematic presentation of the time points and intervals recommended by ISHLT DCD Working Group (12).
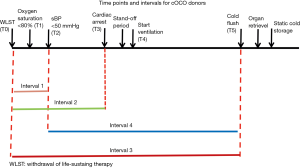
The intervals of times in Figure 1 were defined as: T0 to T2 (interval 1), T0 to T3 (interval 2), T0 to T5 (interval 3) and T2 to T5 (interval 4) (12).
Donor selection criteria
cDCD donors
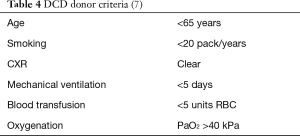
For cDCD donor selection, most of the centers apply internationally agreed DBD donor criteria (Table 4) (7).
Extended criteria donors such as age >65 years, smoking history of >20 pack/years, ICU stay >5 days, and abnormal chest X-ray are accepted in some programs (7). Significant aspiration and a PaO2/FiO2 <300 mmHg are generally not accepted for DCD donation (7,15).
Important issues in clinical DCD practice
- Pre-mortem heparin use;
- Pre-mortem bronchoscopy;
- Placement of nasogastric tube;
- Stand-off period;
- Length of agonal phase;
- Withdrawal of tracheal tube;
- Maximal length of initial warm ischemic period;
- Timing of re-ventilation;
- Selective use of EVLP.
Pre-mortem interventions in a patient who is a potential DCD donor vary widely among the centers due to ethical considerations (27,56-58).
In a patient who is not declared a donor until death, appropriate and maximum treatment of the patient should be continued (7). The other issue is to protect the organ for good outcomes after transplantation. Lung protective ventilation that reduces lung injury (i.e., a tidal volume of 6–8 mL/kg ideal body weight, with PEEP of 8 cmH2O, frequent suctioning) is recommended (7). A pre-mortem bronchoscopy is generally performed among the centers (17,21,23,59,60) to assess the airways and the placement of a nasogastric tube to prevent aspiration of gastric contents (17,59). The airways of a potential DCD donor might be protected from aspiration by omitting extubation; on the other hand, it might prolong the agonal phase by preventing collapse of upper airway of the potential donor (7).
In a pig DCD model Sanchez et al. showed that pre-arrest heparin administration improved organ function by preserving endothelial homeostasis (61). Contrary to this report, Keshava et al. demonstrated that DCD lungs could be used regardless of ante-mortem heparin administration (62). To date there is no clinical study to compare pre-mortem heparin use versus no heparin use. There are some centers that use pre-mortem heparin in a potential DCD donor (17,20,23,24,28,60,63). However some centers do not use premortem heparin (19,21,26,59).
Agonal phase is defined as the time period between withdrawal of life support and cardiac arrest. Although there is not a consensus about the optimal time period among the centers, this period varies from 30 to 180 minutes (17,19-21,23,25,26,59,60,63,64). Most of the centers are allowing maximum time of 90 minutes.
Tolerable WIT, defined as the time between cardiac arrest and cold flush, is around 30 minutes (5,7,10-12,17,19-26,28,59,60,63,64). However, based on experimental data WIT of 60 minutes is tolerable (1,7).
EVLP
The EVLP is as a technology to evaluate and recondition lung graft before transplantation (10,16,58,65). Originally, EVLP has been proposed to assess the function of the lung from an uncontrolled DCD donor (category II) as an interim evaluation of the graft prior to transplantation (58). The Toronto Group modified this method and published their results in nine cDCD donors (66). Selective use of EVLP is a part of the DCD Program in most centers (20,59,66).
The exact role of EVLP in category III DCD has not been established (67). Excellent results have been obtained without the routine use of EVLP (17). In contrast, EVLP may help to exclude lungs with injuries that have not been recognized after withdrawal of life support therapies and may help for acceptance of longer agonal times (67).
uDCD donors
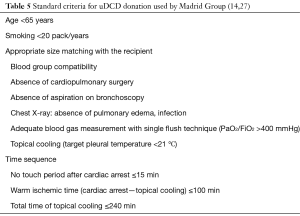
Steen et al. in Sweden performed the first successful LTx from an uDCD after evaluation with EVLP (58). The Madrid Group is the center with the largest experience on uDCD donation (14,27). Standard criteria for uDCD donation used by Madrid Group are shown in Table 5 (14,27).
Madrid Group recently reported 29 lung transplants from uDCD donors (category II) (14). Overall hospital mortality rate was 17%. Survival rates at 1, 2 and 5 years were 68%, 57% and 51%, respectively. The cumulative incidence of bronchiolitis obliterans syndrome (BOS) was 11%, 35% and 45% at 1, 3 and 5 years, respectively (14). Use of EVLP in uDCD donors is strongly recommended (68).
Selective EVLP use in uDCD donors is suggested from Spanish Group according to the following situations (14):
- PaO2/FiO2 <400 mmHg;
- Signs of pulmonary edema on chest X-ray or during procurement;
- Poor lung compliance at the procurement;
- Donors: >65 years old, questionable history of aspiration, heavy smoker, expected long ischemic time.
Outcomes from cDCD donor LTx
Levvey et al. reported 5-year results of 72 category III DCD LTx reported to the Australian National DCD Lung Transplant Collaborative (17). One- and 5-year actuarial survival was 97% and 90% in DCD, vs. 90% and 61%, for 503 DBD lung transplants, respectively (17).
Recently, Leuven Group updated their DCD LTx series in 59 recipients (56). The comparison was done with a cohort of DBD LTx recipients (n=331). There was no difference in time on mechanical ventilation, ICU stay, highest PGD score and hospital stay. Moreover, chronic lung allograft dysfunction (CLAD)-free and overall survival did not differ between the DBD and DCD group (56).
Erasmus et al. from Groningen evaluated the effectiveness of DCD LTx from 35 category III DCD donors (19). Five-year survival was 73% in DCD and 66% in DBD cohorts. Survival, occurrence of PGD, and acute rejection was comparable to the DBD cohort. The incidence of BOS was lower in the DCD group (19).
Mason et al. using data from the United Network for Organ Sharing (UNOS) for LTx compared (I) survival after LTx of recipients of DCD versus DBD donor organs in the United States and (II) recipient characteristics (24). Among 14,939 transplants that were performed, 36 were DCD. Unadjusted survival at 1, 6, 12, and 24 months was 94%, 94%, 94%, and 87%, respectively, for DCD donors versus 92%, 84%, 78%, and 69%, respectively, for DBD donors (P=0.04).
De Oliveira et al. from University of Wisconsin showed that the long-term patient and graft survival rates after DCD LTx were equivalent to those after DBD LTx (60).
St. Louis Group also reported that at their center, early outcomes after DCD LTx were reported to be somewhat inferior to those of series from other centers but approach national averages for conventional LTx (21).
Data from the ISHLT DCD Registry was recently published (12). There were 306 transplants performed using DCD donors and 3,992 transplants using DBD donors during the study period. Median age for DCD donors was 44 years (range, 16–62 years) and 40 years (range, 15–64 years) for DBD donors. Heparin was given in 54% of the cases, donor extubation occurred in 90% of the cases, and selective normothermic EVLP was used in 12%. The median time from withdrawal of life support therapy (WLST) to cardiac arrest was 15 minutes (5th to 95th percentiles of 5 to 55 minutes), and from WLST to cold flush was 33 minutes (5th to 95th percentiles of 19.5 to 79.5 minutes). Thirty-day survival was 96% in the DCD group and 97% in the DBD group. One-year survival was 89% in the DCD group and 88% in the DBD group. Five-year survival was 61% in both groups (12). In order to standardize the definitions around important times in DCD donation process, ISHLT DCD Working Group recommended the following times points and intervals (12) (Table 3, Figure 1). No differences in 1-year survival were observed for the different lengths of intervals 1 and 2 (<10 vs. 10 to 20 vs. 420 minutes; P=0.36 and P=0.83 for intervals 1 and 2, respectively). Similarly, no differences in survival were observed for interval 3 duration (<30 vs. 30 to 45 vs. 445 minutes; P=0.11). There was no significant correlation between the interval of WLST to pulmonary flush with survival (P=0.11) (12).
Recently, Sabashnikov et al. from Harefield investigated long-term outcomes after LTx with DCD donors in comparison with those obtained from DBD donors (64). There were no significant differences regarding intraoperative variables and total ischemic time. Patients from the DCD group had significantly higher incidence of primary graft dysfunction grade 3 at the end of the procedure (P=0.014), and significantly lower PaO2/FiO2 ratio during the first 24 h after the procedure (P=0.018). There was a trend towards higher incidence of the need for postoperative extracorporeal life support in the DCD group. While the overall cumulative survival was not significantly different, the DCD group had significantly poorer results in terms of BOS-free survival in the long-term follow-up (64). They concluded that long-term results after LTx from DCD are in general comparable with those obtained after DBD LTx. However, patients transplanted using organs from DCD donors have a predisposition for development of BOS in the longer follow-up (64).
DCD category III LTx program in Switzerland
Following the legal regulations, utilization of DCD (category III) donors is allowed in Switzerland (1st September 2011). SwissTransplant Working Group on DCD organized multiple meetings. Zurich University Hospital constituted a working group for multiorgan DCD Program. According to our local committee (DCD Working Group) in Zurich, we decided to perform first three DCD category III donors only for kidneys, 4th and 5th for liver, followed by lung retrieval. We performed the first lung DCD LTx in February 2012. As of April 2017, we performed 21 LTxs from DCD donors. Zurich DCD LTx Program details are given in Table 6. We presented the results of the first 19 cases at ISHLT 37th Annual Meeting and Scientific Sessions in San Diego, USA, in April 2017 (69).
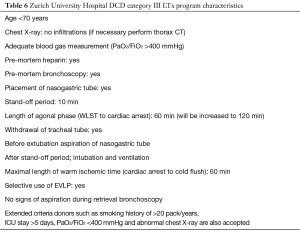
Full table
In our series, median agonal phase (withdrawal-cardiac arrest) was 17 minutes [interquartile range (IQR), 11–20 minutes]. Median donor oxygenation capacity was 48 kPa (IQR, 40–52 kPa). Median WIT (cardiac arrest-cold perfusion) was 31 minutes (IQR, 24–37 minutes). Intraoperative extracorporeal membrane oxygenation (ECMO) was used in seven recipients, two of them were bridged to transplantation on ECMO. In two DCDs normothermic ex vivo lung perfusion was done before implantation. The median intubation time was 1 day (IQR, 1–2 days). ICU time was 3 days (IQR, 2–5 days). Two patients developed primary graft dysfunction grade 3 within 72 h. The 90-day mortality in DCD group was 0%. Actuarial survival rates at 1 and 3 years are 100% and 79% for DCD and 85% and 67% for the DBD group, respectively (P=0.5).
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Van Raemdonck DE, Rega FR, Neyrinck AP, et al. Non-heart-beating donors. Semin Thorac Cardiovasc Surg 2004;16:309-21. [Crossref] [PubMed]
- Egan TM. Non-heart-beating donors in thoracic transplantation. J Heart Lung Transplant 2004;23:3-10. [Crossref] [PubMed]
- Thuong M, Ruiz A, Evrard P, et al. New classification of donation after circulatory death donors definitions and terminology. Transpl Int 2016;29:749-59. [Crossref] [PubMed]
- Reeb J, Keshavjee S, Cypel M. Successful lung transplantation from a donation after cardiocirculatory death donor taking more than 120 minutes to cardiac arrest after withdrawal of life support therapies. J Heart Lung Transplant 2016;35:258-9. [Crossref] [PubMed]
- Mooney JJ, Hedlin H, Mohabir PK, et al. Lung Quality and Utilization in Controlled Donation After Circulatory Determination of Death Within the United States. Am J Transplant 2016;16:1207-15. [Crossref] [PubMed]
- Iyer A, Chew HC, Gao L, et al. Pathophysiological Trends During Withdrawal of Life Support: Implications for Organ Donation After Circulatory Death. Transplantation 2016;100:2621-9. [Crossref] [PubMed]
- Erasmus ME, van Raemdonck D, Akhtar MZ, et al. DCD lung donation: donor criteria, procedural criteria, pulmonary graft function validation, and preservation. Transpl Int 2016;29:790-7. [Crossref] [PubMed]
- Dark JH, Egan TM. Lungs From the Controlled Donation After Circulatory Determination of Death Donor: Perspectives From the United States and Beyond. Am J Transplant 2016;16:1047-8. [Crossref] [PubMed]
- Dark JH. Lung transplantation from donation after cardiocirculatory death: the end of the golden era? Eur J Cardiothorac Surg 2016;49:53-4. [Crossref] [PubMed]
- Reeb J, Keshavjee S, Cypel M. Expanding the lung donor pool: advancements and emerging pathways. Curr Opin Organ Transplant 2015;20:498-505. [Crossref] [PubMed]
- Krutsinger D, Reed RM, Blevins A, et al. Lung transplantation from donation after cardiocirculatory death: a systematic review and meta-analysis. J Heart Lung Transplant 2015;34:675-84. [Crossref] [PubMed]
- Cypel M, Levvey B, Van Raemdonck D, et al. International Society for Heart and Lung Transplantation Donation After Circulatory Death Registry Report. J Heart Lung Transplant 2015;34:1278-82. [Crossref] [PubMed]
- Cypel M, Keshavjee S. Strategies for safe donor expansion: donor management, donations after cardiac death, ex-vivo lung perfusion. Curr Opin Organ Transplant 2013;18:513-7. [Crossref] [PubMed]
- Gomez-de-Antonio D, Campo-Canaveral JL, Crowley S, et al. Clinical lung transplantation from uncontrolled non-heart-beating donors revisited. J Heart Lung Transplant 2012;31:349-53. [Crossref] [PubMed]
- Wigfield CH, Love RB. Donation after cardiac death lung transplantation outcomes. Curr Opin Organ Transplant 2011;16:462-8. [Crossref] [PubMed]
- Cypel M, Yeung JC, Keshavjee S. Novel approaches to expanding the lung donor pool: donation after cardiac death and ex vivo conditioning. Clin Chest Med 2011;32:233-44. [Crossref] [PubMed]
- Levvey BJ, Harkess M, Hopkins P, et al. Excellent clinical outcomes from a national donation-after-determination-of-cardiac-death lung transplant collaborative. Am J Transplant 2012;12:2406-13. [Crossref] [PubMed]
- Aigner C, Slama A, Hotzenecker K, et al. Clinical ex vivo lung perfusion--pushing the limits. Am J Transplant 2012;12:1839-47. [Crossref] [PubMed]
- Van De Wauwer C, Verschuuren EA, van der Bij W, et al. The use of non-heart-beating lung donors category III can increase the donor pool. Eur J Cardiothorac Surg 2011;39:e175-80; discussion e80.
- De Vleeschauwer SI, Wauters S, Dupont LJ, et al. Medium-term outcome after lung transplantation is comparable between brain-dead and cardiac-dead donors. J Heart Lung Transplant 2011;30:975-81. [Crossref] [PubMed]
- Puri V, Scavuzzo M, Guthrie T, et al. Lung transplantation and donation after cardiac death: a single center experience. Ann Thorac Surg 2009;88:1609-14; discussion 14-5. [Crossref] [PubMed]
- De Vleeschauwer S, Van Raemdonck D, Vanaudenaerde B, et al. Early outcome after lung transplantation from non-heart-beating donors is comparable to heart-beating donors. J Heart Lung Transplant 2009;28:380-7. [Crossref] [PubMed]
- Cypel M, Sato M, Yildirim E, et al. Initial experience with lung donation after cardiocirculatory death in Canada. J Heart Lung Transplant 2009;28:753-8. [Crossref] [PubMed]
- Mason DP, Thuita L, Alster JM, et al. Should lung transplantation be performed using donation after cardiac death? The United States experience. J Thorac Cardiovasc Surg 2008;136:1061-6. [Crossref] [PubMed]
- Mason DP, Murthy SC, Gonzalez-Stawinski GV, et al. Early experience with lung transplantation using donors after cardiac death. J Heart Lung Transplant 2008;27:561-3. [Crossref] [PubMed]
- Zych B, Popov AF, Amrani M, et al. Lungs from donation after circulatory death donors: an alternative source to brain-dead donors? Midterm results at a single institution. Eur J Cardiothorac Surg 2012;42:542-9. [Crossref] [PubMed]
- de Antonio DG, Marcos R, Laporta R, et al. Results of clinical lung transplant from uncontrolled non-heart-beating donors. J Heart Lung Transplant 2007;26:529-34. [Crossref] [PubMed]
- Hernadez-Alejandro R, Wall W, Jevnikar A, et al. Organ donation after cardiac death: donor and recipient outcomes after the first three years of the Ontario experience. Can J Anaesth 2011;58:599-605. [Crossref] [PubMed]
- Hardy JD, Webb WR, Dalton ML Jr, et al. Lung Homotransplantation in Man. JAMA 1963;186:1065-74. [Crossref] [PubMed]
- Toronto Lung Transplant Group. Unilateral lung transplantation for pulmonary fibrosis. N Engl J Med 1986;314:1140-5. [Crossref] [PubMed]
- Egan TM, Lambert CJ Jr, Reddick R, et al. A strategy to increase the donor pool: use of cadaver lungs for transplantation. Ann Thorac Surg 1991;52:1113-20; discussion 20-1. [Crossref] [PubMed]
- D'Armini AM, Roberts CS, Griffith PK, et al. When does the lung die? I. Histochemical evidence of pulmonary viability after "death". J Heart Lung Transplant 1994;13:741-7. [PubMed]
- D'Armini AM, Tom EJ, Roberts CS, et al. When does the lung die? Time course of high energy phosphate depletion and relationship to lung viability after "death". J Surg Res 1995;59:468-74. [Crossref] [PubMed]
- Ulicny KS Jr, Egan TM, Lambert CJ Jr, et al. Cadaver lung donors: effect of preharvest ventilation on graft function. Ann Thorac Surg 1993;55:1185-91. [Crossref] [PubMed]
- Egan TM, Ulicny KS Jr, Lambert CJ Jr, et al. Effect of a free radical scavenger on cadaver lung transplantation. Ann Thorac Surg 1993;55:1453-9. [Crossref] [PubMed]
- Roberts CS, Hennington MH, D'Armini AM, et al. Donor lungs from ventilated cadavers: impart of a free radical scavenger. J Heart Lung Transplant 1996;15:275-82. [PubMed]
- Hennington MH, D'Armini AM, Lemasters JJ, et al. Cadaver lungs for transplantation. Effect of ventilation with alveolar gas. Transplantation 1996;61:1009-14. [Crossref] [PubMed]
- Rega FR, Wuyts WA, Vanaudenaerde BM, et al. Nebulized N-acetyl cysteine protects the pulmonary graft inside the non-heart-beating donor. J Heart Lung Transplant 2005;24:1369-77. [Crossref] [PubMed]
- Rega FR, Jannis NC, Verleden GM, et al. Should we ventilate or cool the pulmonary graft inside the non-heart-beating donor? J Heart Lung Transplant 2003;22:1226-33. [Crossref] [PubMed]
- Rega FR, Vanaudenaerde BM, Wuyts WA, et al. IL-1beta in bronchial lavage fluid is a non-invasive marker that predicts the viability of the pulmonary graft from the non-heart-beating donor. J Heart Lung Transplant 2005;24:20-8. [Crossref] [PubMed]
- Dougherty JC, Sinha S, Kibble F, et al. Intolerance of the ischemic lung to hypothermic ventilation. J Appl Physiol 1972;32:632-4. [PubMed]
- Watanabe S, Sakasegawa K, Shimokawa S, et al. Intrathoracic cooling of cadavers before lung transplantation using cold air: an experimental study. Transplantation 2002;73:39-43. [Crossref] [PubMed]
- Steen S, Ingemansson R, Budrikis A, et al. Successful transplantation of lungs topically cooled in the non-heart-beating donor for 6 hours. Ann Thorac Surg 1997;63:345-51. [Crossref] [PubMed]
- Steen S, Liao Q, Wierup PN, et al. Transplantation of lungs from non-heart-beating donors after functional assessment ex vivo. Ann Thorac Surg 2003;76:244-52; discussion 52. [Crossref] [PubMed]
- Yoshida S, Sekine Y, Shinozuka N, et al. The efficacy of partial liquid ventilation in lung protection during hypotension and cardiac arrest: preliminary study of lung transplantation using non-heart-beating donors. J Heart Lung Transplant 2005;24:723-9. [Crossref] [PubMed]
- Okazaki M, Date H, Inokawa H, et al. Optimal time for post-mortem heparinization in canine lung transplantation with non-heart-beating donors. J Heart Lung Transplant 2006;25:454-60. [Crossref] [PubMed]
- Inci I, Zhai W, Arni S, et al. Fibrinolytic treatment improves the quality of lungs retrieved from non-heart-beating donors. J Heart Lung Transplant 2007;26:1054-60. [Crossref] [PubMed]
- Inci I, Arni S, Inci D, et al. Impact of topical cooling solution and prediction of pulmonary graft viability from non-heart-beating donors. J Heart Lung Transplant 2008;27:1016-22. [Crossref] [PubMed]
- Inci I, Arni S, Acevedo C, et al. Surfactant alterations following donation after cardiac death donor lungs. Transpl Int 2011;24:78-84. [Crossref] [PubMed]
- Inci I, Hillinger S, Arni S, et al. Reconditioning of an injured lung graft with intrabronchial surfactant instillation in an ex vivo lung perfusion system followed by transplantation. J Surg Res 2013;184:1143-9. [Crossref] [PubMed]
- Martens A, Boada M, Vanaudenaerde BM, et al. Steroids can reduce warm ischemic reperfusion injury in a porcine donation after circulatory death model with ex vivo lung perfusion evaluation. Transpl Int 2016;29:1237-46. [Crossref] [PubMed]
- Huerter ME, Sharma AK, Zhao Y, et al. Attenuation of Pulmonary Ischemia-Reperfusion Injury by Adenosine A2B Receptor Antagonism. Ann Thorac Surg 2016;102:385-93. [Crossref] [PubMed]
- Kootstra G, Daemen JH, Oomen AP. Categories of non-heart-beating donors. Transplant Proc 1995;27:2893-4. [PubMed]
- Levvey BJ, Westall GP, Kotsimbos T, et al. Definitions of warm ischemic time when using controlled donation after cardiac death lung donors. Transplantation 2008;86:1702-6. [Crossref] [PubMed]
- Snell GI, Oto T, Levvey B, et al. Evaluation of techniques for lung transplantation following donation after cardiac death. Ann Thorac Surg 2006;81:2014-9. [Crossref] [PubMed]
- Ruttens D, Martens A, Ordies S, et al. Short- and Long-Term Outcome After Lung Transplantation From Circulatory-Dead Donors: A Single-Center Experience. Transplantation 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Snell GI, Levvey BJ, Oto T, et al. Early lung transplantation success utilizing controlled donation after cardiac death donors. Am J Transplant 2008;8:1282-9. [Crossref] [PubMed]
- Steen S, Sjoberg T, Pierre L, et al. Transplantation of lungs from a non-heart-beating donor. Lancet 2001;357:825-9. [Crossref] [PubMed]
- Erasmus ME, Verschuuren EA, Nijkamp DM, et al. Lung transplantation from nonheparinized category III non-heart-beating donors. A single-centre report. Transplantation 2010;89:452-7. [Crossref] [PubMed]
- De Oliveira NC, Osaki S, Maloney JD, et al. Lung transplantation with donation after cardiac death donors: long-term follow-up in a single center. J Thorac Cardiovasc Surg 2010;139:1306-15. [Crossref] [PubMed]
- Sanchez PG, Bittle GJ, Williams K, et al. Ex vivo lung evaluation of prearrest heparinization in donation after cardiac death. Ann Surg 2013;257:534-41. [Crossref] [PubMed]
- Keshava HB, Farver CF, Brown CR, et al. Timing of heparin and thrombus formation in donor lungs after cardiac death. Thorac Cardiovasc Surg 2013;61:246-50. [Crossref] [PubMed]
- Mason DP, Brown CR, Murthy SC, et al. Growing single-center experience with lung transplantation using donation after cardiac death. Ann Thorac Surg 2012;94:406-11; discussion 11-2. [Crossref] [PubMed]
- Sabashnikov A, Patil NP, Popov AF, et al. Long-term results after lung transplantation using organs from circulatory death donors: a propensity score-matched analysisdagger. Eur J Cardiothorac Surg 2016;49:46-53. [Crossref] [PubMed]
- Yeung JC, Cypel M, Waddell TK, et al. Update on donor assessment, resuscitation, and acceptance criteria, including novel techniques--non-heart-beating donor lung retrieval and ex vivo donor lung perfusion. Thorac Surg Clin 2009;19:261-74. [Crossref] [PubMed]
- Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011;364:1431-40. [Crossref] [PubMed]
- Cypel M, Levvey B, Van Raemdonck D, et al. Lung transplantation using controlled donation after circulatory death donors: Trials and tribulations. J Heart Lung Transplant 2016;35:146-7. [Crossref] [PubMed]
- Egan TM, Requard JJ 3rd. Uncontrolled Donation After Circulatory Determination of Death Donors (uDCDDs) as a Source of Lungs for Transplant. Am J Transplant 2015;15:2031-6. [Crossref] [PubMed]
- Inci I, Lenherr R, Hillinger S, et al. Lung Transplantation with Controlled Donation After Circulatory Death Donors: Initial Experience in Switzerland. J Heart Lung Transplant 2017;36:S319. [Crossref]