Management of cardiac implantable electronic devices during interventional pulmonology procedures
Introduction
Bronchoscopy is a safe procedure with most studies reporting a low complication rate between 0.08–1.7% (1-6). A collaborative study by the American Society of Gastrointestinal Endoscopy and United States Food and Drug Administration estimated that majority of serious complications and mortalities during endoscopy procedures occur in the patients with preexisting cardiopulmonary diseases (7). Arrhythmias constitute a significant percent of the cardiovascular complications and have largely been attributed to premedication, procedural anesthesia, hypoxia, abnormal autonomic activity and myocardial ischemia.
Cardiac implantable electronic devices (CIEDs) including permanent pacemakers (PPM), implantable cardioverter defibrillators (ICD) and cardiac resynchronization therapy (CRT) devices are used to treat various cardiac dysrhythmias, prevention of sudden cardiac death and for treatment of heart failure (8,9). Trends of device implantation for the CIEDs show a steady increase in the number of procedures being performed (10,11). Eighty-five percent of the patients with pacemakers have a significant number of comorbidities and are therefore at increased risk of complications even during minimally invasive endoscopic procedures (12).
Over the last two decades, interventional pulmonology (IP) has evolved considerably to include a range of technologies. Many IP modalities such as Laser, Electrocautery, Argon Plasma Coagulation and Electromagnetic Navigational Bronchoscopy generate electromagnetic field of varying strength and have been anecdotally associated with inadvertent interactions with CIEDs. Consequently, their safety and feasibility has been questioned in patients with these devices. Our goal here is to review and summarize the data pertaining to the management of CIED related issues in patients being considered for these interventions.
Glossary of CIED terminology relevant to interactions with IP procedures
Pacemaker: a device capable of providing electrical stimulation to the heart (pacing). Contemporary pacemakers also detect intrinsic cardiac electrical activity (sensing).
Pacemaker types: a single chamber pacemaker has a single lead and could be an atrial or more commonly a ventricular pacemaker. A dual chamber pacemaker has leads both in the atrium and the ventricle.
ICD: a device capable of detecting and terminating ventricular tachyarrhythmias (tachycardia/fibrillation) by delivering a high-energy electrical shock. ICDs with transvenous leads in the heart also have pacing capability. ICDs can be single or dual chamber, or can be standalone entirely subcutaneous systems without any endocardial leads.
CRT: a pacing strategy to electrically resynchronize the dyssynchronous left ventricular activation that often occurs in the setting of left bundle branch block. This is usually delivered by synchronously pacing from one site within the right ventricle and another site on the left ventricular free wall often with a lead implanted in a coronary vein. CRT can be delivered by a device also capable of defibrillation (defibrillator, CRT-D), or lacking defibrillation capability (pacemaker, CRT-P).
Pacemaker modes: CIED programming that dictates pacemaker behavior. An asynchronous mode paces indiscriminately at the programmed rate without assessing for any intrinsic cardiac activity. An inhibitory mode delivers pacing impulses only in the absence of intrinsic cardiac activity faster than the programmed pacing rate. A triggered mode delivers a pacing impulse as a response to a sensed cardiac event, for example triggered or tracked ventricular pacing after an appropriate offset (atrioventricular delay) in response to intrinsic atrial activity.
Electromagnetic interference (EMI): an electrical signal generated by conduction, electromagnetic induction or electrostatic coupling from an external source that falls within the sensing frequency spectrum of the CIED.
Oversensing: spurious detection by CIED of a cardiac event in the absence of any true physiological myocardial electrical activation, for example inappropriate detection of EMI by CIED as cardiac activity.
Unipolar versus bipolar sensing: a CIED lead is in a bipolar configuration if the sensing circuit records between two relatively closely spaced electrodes close to the tip of the lead. In a unipolar configuration, the sensing circuit records between tip electrode and the CIED generator. The wide spacing of the sensing electrodes in a unipolar configuration makes it prone to oversensing EMI, as opposed to bipolar configuration that limits the sensing to electrical activity generated from the near-field myocardium.
Inhibition: suppression of pacing due to sensing of electrical activity. Appropriate inhibition occurs due to presence of intrinsic cardiac activity. Inappropriate inhibition can occur due to oversensing of EMI potentially leading to asystole in pacemaker dependent patients.
Automatic mode switch: dual chamber CIEDs are commonly programmed in a tracking mode to enable ventricular pacing after every intrinsic atrial complex so as to maintain atrioventricular synchrony during sinus rhythm. However, tracking is not desirable during atrial tachyarrhythmias like atrial fibrillation as this would result in inappropriate rapid ventricular pacing. CIEDs thus automatically switch to a non-tracking mode when atrial activity faster than a programmed cutoff (usually 150–200 per minute) is detected.
Noise reversion mode: in modern CIEDs, detection of non-physiologic high frequency EMI results in a noise reversion mode with asynchronous pacing to prevent inappropriate inhibition. Further, CIEDs may have algorithms to prevent inappropriate detection of ventricular tachyarrhythmia. Asynchronous pacing may sometimes induce tachyarrhythmias due to potential for pacing during the vulnerable period after intrinsic electrical activity (R on T phenomenon).
Pacemaker reset: powerful external signals may reset the CIED to a “power-on” basic factory preset mode stored on the non-volatile read-only memory.
Inappropriate shock: a shock delivered by an ICD due to inappropriate detection of ventricular tachyarrhythmia due to oversensing. This may occur due to EMI, oversensing of extracardiac physiologic signals (for example diaphragmatic myopotentials) or CIED lead malfunction.
Specific IP procedures
Light amplification by stimulated emission of radiation (LASER)
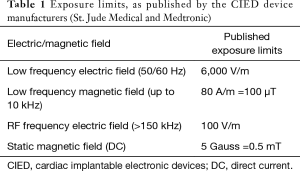
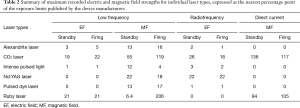
Laser was first used in medicine in the 1960’s (13-15). It is one of the most commonly used ablative modalities in the management of malignant and nonmalignant airway lesions. Six different types of laser systems are available today for medical use, ranging in wavelength between 516–10,600 nm and in penetration between 0.23–10 mm (16). Neodymium-Yttrium, Aluminum, Garnet (Nd-YAG) laser, with the wavelength of 1,064 nm, is the most commonly used laser bronchoscopically. The coagulation effect and cutting effect of individual laser systems determines their suitability for the procedure being planned. Hemorrhage, endobronchial fire, pneumothorax, pneumomediastinum and air embolism are some of the common complications. While cardiovascular complications have not been frequently reported, concerns of EMI have been raised in patients with CIEDs. In a study by Lister et al., the electromagnetic field strength around the six laser systems was measured in a laboratory suite and compared against the exposure limits specified by the cardiac device manufacturers (Medtronic and St. Jude Medical). Published exposure limits for the CIEDs applied in this study are included in Table 1. Comparative safety of the various laser systems during standby vs firing modes are summarized in Table 2. All except the CO2 and ruby lasers were found to generate electromagnetic field strengths below the permissible limits for CIEDs (17). A similar Brazilian in vitro study in 2015 assessed the EMI between the CIEDs and the endodontic laser devices and reported no interference caused by the endodontic TwinFlex laser (wavelength range 660–808 nm) device (18). Another study evaluating the in vitro EMI between the CIEDs and ophthalmic laser systems (wavelength range 193–1,064 nm) concluded that the CIEDs were not affected by the EMI generated by the ophthalmic laser systems (19). In general, laser appears to be safe to use from the EMI point of view in these in vitro studies.
Full table
Full table
Electrocautery
Endobronchial electrocautery through flexible bronchoscope was first reported in early 1980’s (20-24). It is a contact electrosurgical ablation method in which the heat generated by the passage of high frequency electric current (radiofrequency range 100–5,000 kHz) from the probe to tissues causes coagulation or vaporization of the target tissue. There are two main configurations of electrocautery current delivery: monopolar and bipolar. In monopolar configuration, current is delivered through a cauterizing instrument and exits through a “ground” or “return” electrode placed somewhere on the patient body, usually the leg. Current travels through a large amount of tissue thereby generating significant EMI. The impact of this EMI on CIEDs is influenced by proximity as well as the orientation of the electrodes with respect to the CIED and its leads (25). In bipolar configuration, both the entry and exit electrodes are together at the tip of the cauterizing instrument and hence the EMI field is too small to impact functioning of the CIEDs.
Most of the problems with CIEDs are reported while using electrocautery in the monopolar configuration (26,27). CIEDs can misinterpret the non-physiologic radiofrequency signals from EMI as pathological intracardiac events and consequently behave in undesirable ways. CIED behavior related to EMI includes inhibition of pacing, triggered pacing, automatic mode switching, pacemaker reset or reprogramming and noise reversion (28-30). ICDs can spuriously interpret the EMI signals as ventricular tachyarrhythmia and deliver inappropriate shocks (31,32). The high energy delivered by the electrocautery to the body can also damage the CIED generator and cause permanent malfunction. Lastly, the CIED leads can route the applied electrocautery energy or transmit inducted electrical energy through the electrode-myocardial interface potentially resulting in electrical cardiac stimulation or thermal injury.
Argon plasma coagulation (APC)
APC was introduced in open surgery in 1977 but first used endoscopically only in 1991 (33-35). It is a non-contact electrosurgical ablation modality used for homogeneous and superficial tissue coagulation. Current flows from a probe to the target tissue and exits through a return electrode placed usually on patient’s leg. Plasma in APC refers to the ionized argon gas jet flow. The distance between the electrode probe and the tissue to be treated is 2–10 mm (usually 3–5 mm). The plasma flows circumferentially around the electrode contained in a tube, and is ignited by the alternating current voltage (typically with amplitude of 4 kV and frequency of 350 kHz) through the electrode. The probe should be pushed 5–10 mm beyond the bronchoscope tip to avoid concurrent scope damage and supplemental oxygen be kept at ≤40% to prevent fire. Characteristics such as biochemical inertness, low breakdown voltage, operational simplicity and low cost make it a preferred intervention for endoscopic tissue ablation. The major advantages of APC over laser are in ablating distal/apical lesions and controlling hemoptysis (36). Complications reported with APC are gas embolism, airway fire, pneumomediastinum, pneumothorax, subcutaneous emphysema and burnt bronchoscopes.
Due to the monopolar circuitry and high voltage use, APC creates high risk EMI with the CIEDs. In a study that measured EMI of multiple energy-based devices on ICD, EMI from APC was measured at 2.58±0.34 mV and classified as “high risk” (37). Similar to electrocautery, EMI can result in inhibition of pacing, device reset, inappropriate shock or damage to the CIED circuitry.
Electromagnetic navigational bronchoscopy
First reported in swine models in 2003, the earliest human experience with Electromagnetic navigational bronchoscopy (ENB) was published in 2006 as an investigational procedure for biopsy of peripheral lung lesions (38-40). It entails a planning phase where a thin slice chest CT scan is obtained which is analyzed by the software to create a virtual pathway to the lesion. A board placed underneath the patient then creates an electromagnetic field around the subject that guides the sensor device (locatable guide) to the target lesion.
An in vitro study measured the electromagnetic field used by ENB at <0.0001 tesla, similar to the earth’s gravity. This study failed to show any CIED dysfunction due to EMI caused by the ENB field. However, the human models used in the study were not replicative of the real-life circumstances (41). Because of this electromagnetic field, a theoretical risk of EMI causing interaction with CIEDs has been postulated. In fact, ENB is relatively contraindicated in patients with CIEDs per the 2003 CHEST guidelines for IP procedures (42).
Basic principles of management
General considerations
The current guidelines and consensus statements on the management of CIEDs are mostly based on case reports and small case series (42-45). Since no randomized controlled trials have addressed EMI in patients with CIED, many recommendations are based solely on expert opinion. All the guidelines unequivocally stress on individualized management for each patient depending on their comorbidities and risk factors. The most important aspect of pre-procedure management is an active communication between the procedure team (bronchoscopist, anesthesiologist and the periprocedural assessment team) and the CIED team (cardiologist, cardiac electrophysiologist and the device nurses and staff).
Although not always necessary, when needed, patient should be scheduled for preoperative evaluation with sufficient time at hand for investigations and medical optimization as needed. Both pacemakers and ICDs should have been interrogated within the last 12 and 6 months prior to the planned procedure, respectively. The Heart Rhythm Society/American Society of Anesthesiologists consensus statement strongly recommends that patient’s primary CIED team provides recommendations on periprocedural management. If that is not feasible, the on-site CIED team provides the care in collaboration with the bronchoscopist. Industry-employed allied professions are not the appropriate personnel to manage CIED in this situation as it is considered well beyond their scope of practice (46). The bronchoscopist should work with the CIED team to clearly identify the procedure type as well as the associated EMI risk. Procedural aspects such as anatomic location, patient position, monopolar cautery use, other potential sources of EMI, plan or risk for cardioversion/defibrillation, procedure setting (endoscopy suite vs operating room), post-procedure disposition (inpatient admission vs same-day discharge) and potential extraordinary circumstances (excessive bleeding risk, procedural field being too close to the CIED, etc.) should be discussed in detail with the CIED team. The CIED team in turn provides a prescription to the procedure team for periprocedural management of the CIED. General principles that underscore this prescription as per the HRS/ASA guidelines include: (I) ICD sensing deactivation is not mandatory; (II) even in pacemaker dependent patients, changing to asynchronous mode is not always necessary; (III) when required, pacemakers may be rendered asynchronous by reprogramming or by placing a magnet on the pulse generator (latter works for ICDs as well); (IV) a universal indication for deactivating ICD sensing is while using monopolar electrocautery or above-umbilicus radiofrequency ablation; and (V) no reprogramming is needed in below-umbilicus use of electrocautery. The last recommendation is irrelevant for bronchoscopists due to the obvious spatial consideration.
Periprocedural risks
The prime risk of EMI with pacemakers is the cessation of pacing in a pacemaker-dependent patient. With ICDs, anti-tachycardia pacing (ATP) or shock administration is the main concern. In CIEDs with a rate response using an electrical impedance sensor, inappropriate rapid pacing may occur. In general, bipolar electrosurgery is not associated with significant EMI causing device malfunction. The HRS/ASA guidelines identify the following potential behaviors with other modalities: oversensing (with consequent inhibition of pacing or inappropriate ICD therapies), device reset, pulse generator damage, and pacing at upper rate behavior by the impendence based rate-response systems. Four factors mainly determine the likelihood and severity of these adverse events: site of surgery, underlying cardiac rhythm, type and programming of the CIED and type of the equipment generating the EMI. In general, the interference decreases as the distance of the procedure from the CIED increases. The largest case series to date showed that the EMI-CIED interaction occurred only when the distance was <8 cm. The underlying cardiac rhythm is important in that only those patients who are completely pacemaker dependent are at risk of severe bradycardia or asystole. EMI lasting <5 seconds in non-dependent patients may cause minor pacing issues but is unlikely to cause asystole.
Basics of magnet use
All CIEDs are fitted with a reed switch consisting of two magnetic metal strips. These strips are normally placed apart in glass capsules but are activated and come in contact when a magnetic field is applied. This contact causes a sudden voltage change that is detected by the sensing amplifier which changes the pacing to asynchronous mode in a pacemaker and for an ICD, prevents detection of ventricular tachyarrhythmia episodes (and thereby administration of shock) during malignant arrhythmias, though magnet response may be programmable in different CIEDs (47). Most industry-provided magnets have the field strength of >90 Gauss (minimum required strength: >10 Gauss). The CIED team may recommend either using the magnet or reprogramming the device but these two must not be considered interchangeable. Using a magnet is advantageous in situations wherein spontaneous malignant tachyarrhythmia occur. As soon as the magnet is removed, the device detects the malignant rhythm and delivers appropriate therapeutic intervention as programmed. Using the magnet also eliminates the risk to the patients posed by inadvertent failure of the CIED team to revert the device back to the original therapeutic settings (48). In a database-based evaluation of ICD patients between 1996 and 2003, 11 ICDs were deactivated (3 after surgery) among the 212 reported deaths (49).
Intra-procedural management
It is universally agreed that all patients with CIEDs undergoing procedures generating electromagnetic field should be on continuous cardiac monitoring during the procedure (43,50). The ECG monitor being used should have a pacing mode capable of recognizing the pacing stimulus. External defibrillators must be available for use in the procedure room. High risk patients may be better served with the defibrillation pads placed prophylactically before initiating the procedure. Additionally, hemodynamics should be monitored using plethysmographic or arterial pressure monitoring for all these patients.
A key determinant of pacemaker management strategy is patient’s pacemaker dependence. As would be expected, pacemaker-dependent patients (by definition, patients likely to suffer from asystole or severe bradycardia in the absence of pacemaker pacing) are at the highest risk of harm. While patients that are not pacemaker dependent may do well without any programming change, asynchronous pacing in pacemaker dependent patients either by magnet application or by reprogramming the mode should be considered if the pulse generator is accessible. Similarly, for patients with ICD, anti-tachycardia therapy/shock is inhibited by the application of the magnet or pre-procedure reprogramming. Inactivating the minute ventilation sensors could also be considered to negate the effects of rate responsive pacing. For patients who need central venous catheter placement using the Seldinger technique, one must be careful inserting the guidewire in the heart especially for the newly placed CIEDs due to risk of mechanical interaction with the CIED leads that may result in lead dislodgement.
Whenever feasible, bipolar electrocautery probes should be preferred as the closely placed electrodes cause very small electromagnetic field and little interaction with the CIEDs. Monopolar configuration causes large EMI and should be avoided. When monopolar electrocautery or APC is used, the return or exit electrode should be placed such that the path of the current is farthest possible from the CIED. The incidence of EMI decreases as the distance of the current from the CIED increases, and in one large series was reported only when the distance of electrocautery use was <8 cm from the CIED. For this reason, the thigh or leg contralateral to the CIED is the preferred location for placement of the exit electrode. The incidence of clinically significant EMI is also directly proportional to the duration and strength of electrical energy used. Short bursts (preferably <5 seconds with 5-second pauses) of lowest strength clinically effective energy are therefore preferred.
Laser therapy creates weaker electromagnetic fields. Many different type of lasers have been shown to generate lower EMI than the permissible threshold for CIEDs. Barring the CO2 and ruby lasers (which are rarely used now), their use could be considered safe in these patients. Although relatively contraindicated per the current CHEST guidelines, ENB procedures generate low-strength electromagnetic field akin to the earth’s gravitational field. A prospective case series published in 2013 did not report any arrhythmia or disruption of CIED functions in 24 patients who underwent ENB (51). Another prospective series from Italy enrolled 13 patients with ICD undergoing ENB and did not report any electromagnetic noise detection. When ventricular tachyarrhythmia was induced as a part of the study protocol, it was promptly detected and treated by the ICDs as programmed (52). Of courses, these papers were published a decade later and did not contribute to the CHEST 2003 guidelines.
Post-procedural management
The HRS/ASA expert consensus statement recommends post-procedure CIED interrogation before moving the patients from a cardiac monitored environment in the following scenarios: CIEDs were re-programmed prior to the procedure; surgeries with serious hemodynamic risks like cardiac or vascular surgeries; significant intraprocedural events including hemodynamic compromise, cardiac arrest, ventricular tachycardia, cardiopulmonary resuscitation, temporary pacing or external defibrillation; site of EMI exposure above the umbilicus; high intensity EMI exposure that poses greater risk to the CIED function (e.g., external cardioversion or radiofrequency ablation); and, in patients with logistical barriers to a reliable follow-up and device evaluation within 1 month from the procedure. Regardless of whether monopolar or bipolar probe was used, all bronchoscopy procedures with potential for EMI generation would qualify for post-procedure CIED interrogation by geographically being located above the umbilicus. It is imperative to ensure that the devices that were re-programmed before the procedure must be reverted to their pre-procedure sensing and therapeutic thresholds. The management strategies for the various CIEDs are summarized in Table 3.

Full table
Conclusions
With the broadening inclusion criteria for patients who might benefit from cardiac implantable devices, the exposure of interventional pulmonologists to these devices is inevitable and ever-growing. Certain IP procedures like electrocautery and APC do create significant EMI and hence, a systematic approach to the patients with CIEDs undergoing these procedures is warranted. Procedures like laser and electromagnetic navigation bronchoscopy conventionally raised concerns of significant EMI but have been reported to be safe lately, as reviewed above. The existing CHEST 2003 guidelines for IP procedures mention ENB as relatively contraindicated in patients with cardiac implantable devices. However, recent literature on ENB safety in these patients would beg for a revision of these guidelines.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Luck JC, Messeder OH, Rubenstein MJ, et al. Arrhythmias from fiberoptic bronchoscopy. Chest 1978;74:139-43. [Crossref] [PubMed]
- Credle WF Jr, Smiddy JF, Elliott RC. Complications of Fiberoptic Bronchoscopy. Am Rev Respir Dis 1974;109:67-72. [PubMed]
- Suratt PM, Smiddy JF, Gruber B. Deaths and complications associated with fiberoptic bronchoscopy. Chest 1976;69:747-51. [Crossref] [PubMed]
- Pereira W, Kovnat DM, Snider GL. A prospective cooperative study of complications following flexible fiberoptic bronchoscopy. Chest 1978;73:813-6. [Crossref] [PubMed]
- Facciolongo N, Patelli M, Gasparini S, et al. Incidence of complications in bronchoscopy. Multicentre prospective study of 20,986 bronchoscopies. Monaldi Arch Chest Dis. 2009;71:8-14. [PubMed]
- Hsu LH, Liu CC, Ko JS, et al. Safety of interventional bronchoscopy through complication review at a cancer center. Clin Respir J 2016;10:359-67. [Crossref] [PubMed]
- Arrowsmith JB, Gerstman BB, Fleischer DE, et al. Results from the American Society for Gastrointestinal Endoscopy/US Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc 1991;37:421-7. [Crossref] [PubMed]
- Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;51:e1-e62. [Crossref] [PubMed]
- Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. Circulation 2013;127:e283-e352. [Crossref] [PubMed]
- Kurtz SM, Ochoa JA, Lau E, et al. Implantation trends and patient profiles for pacemakers and implantable cardioverter defibrillators in the United States: 1993–2006. Pacing Clin Electrophysiol 2010;33:705-11. [Crossref] [PubMed]
- Greenspon AJ, Patel JD, Lau E, et al. Trends in permanent pacemaker implantation in the United States from 1993 to 2009: increasing complexity of patients and procedures. J Am Coll Cardiol 2012;60:1540-5. [Crossref] [PubMed]
- Kalin R, Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol 2005;28:326-8. [Crossref] [PubMed]
- Jako GJ. Laser surgery of the vocal cordsan experimental study with carbon dioxide lasers on dogs. Laryngoscope 1972;82:2204-16. [Crossref] [PubMed]
- Dwyer RM. The Technique of Gastrointestinal Laser Endoscopy. In: Goldman L. editor. The Biomedical Laser. New York: Springer, 1981:255-69.
- Fisher JC. A Brief History of the Nd:YAG Laser. In: Joffe SN, Oguro Y. editors. Advances in Nd:YAG Laser Surgery. New York: Springer, 1988:7-9.
- Khemasuwan D, Mehta AC, Wang KP. Past, present, and future of endobronchial laser photoresection. J Thorac Dis 2015;7:S380-8. [PubMed]
- Lister T, Grant L, Lee SM, et al. Electromagnetic interference from lasers and intense light sources in the treatment of patients with artificial pacemakers and other implantable cardiac devices. Lasers Med Sci 2015;30:1619-22. [Crossref] [PubMed]
- Dadalti MT, da Cunha AJ, de Araújo MC, et al. Electromagnetic interference of endodontic equipments with cardiovascular implantable electronic device. J Dent 2016;46:68-72. [Crossref] [PubMed]
- Sher NA, Golben MP, Kresge K, et al. An in vitro evaluation of electromagnetic interference between implantable cardiac devices and ophthalmic laser systems. Europace 2011;13:583-8. [Crossref] [PubMed]
- Takizawa N, Oho K, Amemiya R, et al. Electrosurgery via the fiberoptic bronchoscope. In: Nakhosteen JA, Maassen W. editors. Bronchology: Research, Diagnostic, and Therapeutic Aspects. Developments in Surgery, vol 3. Dordrecht: Springer, 1981:559-61.
- Taguchi H, Nagata T, Kawai H, et al. High-frequency electrosurgical treatment of tracheal obstruction using the flexible bronchofiberscope. n: Nakhosteen JA, Maassen W. editors. Bronchology: Research, Diagnostic, and Therapeutic Aspects. Developments in Surgery, vol 3. Dordrecht: Springer, 1981:563-5.
- Spinelli P, Pizzetti P, Gullo CL, et al. Resection of obstructive bronchial fibrolipoma through the flexible fiberoptic bronchoscope. Endoscopy 1982;14:61-3. [Crossref] [PubMed]
- Hooper RG, Spratling L, Beechler C, et al. Endobronchial electrocautery. A role in bronchogenic carcinoma? Endoscopy 1984;16:67-70. [Crossref] [PubMed]
- Hooper RG, Jackson FN. Endobronchial electrocautery. Chest 1985;87:712-4. [Crossref] [PubMed]
- Chauvin M, Crenner F, Brechenmacher C. Interaction between permanent cardiac pacing and electrocautery: the significance of electrode position. Pacing Clin Electrophysiol 1992;15:2028-33. [Crossref] [PubMed]
- Levine PA, Balady GJ, Lazar HL, et al. Electrocautery and pacemakers: management of the paced patient subject to electrocautery. Ann Thorac Surg 1986;41:313-7. [Crossref] [PubMed]
- Hemel NM, Hamerlijnck RP, Pronk KJ, et al. Upper limit ventricular stimulation in respiratory rate responsive pacing due to electrocautery. Pacing Clin Electrophysiol 1989;12:1720-3. [Crossref] [PubMed]
- Irnich W, Bakker J, Bisping HJ. Electromagnetic interference in implantable pacemakers. Pacing Clin Electrophysiol 1978;1:52-61. [Crossref] [PubMed]
- Domino KB, Smith TC. Electrocautery-induced reprogramming of a pacemaker using a precordial magnet. Anesth Analg 1983;62:609-12. [Crossref] [PubMed]
- Heller LI. Surgical electrocautery and the runaway pacemaker syndrome. Pacing Clin Electrophysiol 1990;13:1084-5. [Crossref] [PubMed]
- Cheng A, Nazarian S, Spragg DD, et al. Effects of Surgical and Endoscopic Electrocautery on Modern-Day Permanent Pacemaker and Implantable Cardioverter-Defibrillator Systems. Pacing Clin Electrophysiol 2008;31:344-50. [Crossref] [PubMed]
- Guertin D, Faheem O, Ling T, et al. Electromagnetic interference (EMI) and arrhythmic events in ICD patients undergoing gastrointestinal procedures. Pacing Clin Electrophysiol 2007;30:734-9. [Crossref] [PubMed]
- Farin G, Grund K. Technology of argon plasma coagulation with particular regard to endoscopic applications. Endosc Surg Allied Technol 1994;2:71-7. [PubMed]
- Grund KE, Storek D, Farin G. Endoscopic argon plasma coagulation (APC) first clinical experiences in flexible endoscopy. Endosc Surg Allied Technol 1994;2:42-6. [PubMed]
- Morrison CF Jr. Electrosurgical method and apparatus for initiating an electrical discharge in an inert gas flow. Google Patents, 1977.
- Morice RC, Ece T, Ece F, et al. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest 2001;119:781-7. [Crossref] [PubMed]
- Paniccia A, Rozner M, Jones EL, et al. Electromagnetic interference caused by common surgical energy-based devices on an implanted cardiac defibrillator. Am J Surg 2014;208:932-6. [Crossref] [PubMed]
- Schwarz Y, Mehta AC, Ernst A, et al. Electromagnetic navigation during flexible bronchoscopy. Respiration 2003;70:516-22. [Crossref] [PubMed]
- Schwarz Y, Greif J, Becker HD, et al. Real-time electromagnetic navigation bronchoscopy to peripheral lung lesions using overlaid CT images: the first human study. Chest 2006;129:988-94. [Crossref] [PubMed]
- Gildea TR, Mazzone PJ, Karnak D, et al. Electromagnetic navigation diagnostic bronchoscopy: a prospective study. American journal of respiratory and critical care medicine 2006;174:982-9. [Crossref] [PubMed]
- Magnani A, Matheoud R, Brambilla M, et al. In vitro tests of electromagnetic interference of electromagnetic navigational bronchoscopy to implantable cardioverter defibrillators. Europace 2012;14:1054-9. [Crossref] [PubMed]
- Ernst A, Silvestri GA, Johnstone D. Interventional pulmonary procedures: guidelines from the American College of Chest Physicians. Chest 2003;123:1693-717. [Crossref] [PubMed]
- Crossley GH, Poole JE, Rozner MA, et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) expert consensus statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management: this document was developed as a joint project with the American Society of Anesthesiologists (ASA), and in collaboration with the American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Heart Rhythm 2011;8:1114-54. [Crossref] [PubMed]
- Healey JS, Merchant R, Simpson C, et al. Canadian Cardiovascular Society/Canadian Anesthesiologists' Society/Canadian Heart Rhythm Society joint position statement on the perioperative management of patients with implanted pacemakers, defibrillators, and neurostimulating devices. Can J Cardiol 2012;28:141-51. [Crossref] [PubMed]
- Sticherling C, Menafoglio A, Burri H, et al. Recommendations for the perioperative management of patients with cardiac implantable electronic devices. Cardiovascular Medicine 2016;19:13-8. [Crossref]
- Lindsay BD, Estes NM, Maloney JD, et al. Heart Rhythm Society policy statement update: recommendations on the role of industry employed allied professionals (IEAPs). Heart Rhythm 2008;5:e8-e10. [Crossref] [PubMed]
- Jacob S, Panaich SS, Maheshwari R, et al. Clinical applications of magnets on cardiac rhythm management devices. Europace 2011;13:1222-30. [Crossref] [PubMed]
- Rasmussen MJ, Friedman PA, Hammill SC, et al. Unintentional deactivation of implantable cardioverter-defibrillators in health care settings. Mayo Clin Proc 2002;77:855-9. [Crossref] [PubMed]
- Hauser RG, Kallinen L. Deaths associated with implantable cardioverter defibrillator failure and deactivation reported in the United States Food and Drug Administration Manufacturer and User Facility Device Experience Database. Heart Rhythm 2004;1:399-405. [Crossref] [PubMed]
- American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Cardiac Rhythm Management Devices. Practice advisory for the perioperative management of patients with cardiac rhythm management devices: pacemakers and implantable cardioverter-defibrillators: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Cardiac Rhythm Management Devices. Anesthesiology 2005;103:186-98. [Crossref] [PubMed]
- Khan AY, Berkowitz D, Krimsky WS, et al. Safety of pacemakers and defibrillators in electromagnetic navigation bronchoscopy. Chest 2013;143:75-81. [Crossref] [PubMed]
- Magnani A, Balbo P, Facchini E, et al. Lack of interference of electromagnetic navigation bronchoscopy to implanted cardioverter-defibrillator: in-vivo study. Europace 2014;16:1767-71. [Crossref] [PubMed]


