The relationship between apnoea hypopnoea index and Gensini score in patients with acute myocardial infarction undergoing emergency primary percutaneous coronary intervention
Introduction
Sleep apnoea hypopnoea syndrome (SAHS) causes repetitive nocturnal hypoxemia, which could promote the progression of hypertension, arrhythmia, coronary artery disease (CAD) and cerebral vascular disease (1,2). There are many reports regarding the effect of SAHS on high blood pressure (3,4) and arrhythmia (5,6). And most of researches support the opinion that hypertension and arrhythmia with SAHS can be cured or alleviated by continuous positive airway pressure (CPAP) (7-9). But different conclusions are made regarding the effect of SAHS on acute myocardial infarction (AMI), and no reports can be found about the effect of SAHS on AMI for those undergoing emergency primary percutaneous coronary intervention (pPCI).
As we know, the prevalence of AMI is currently increasing, with a trend towards younger-aged patients. Findings from published reports present that the prevalence of SAHS among patients with AMI is up to 60% (10), which is mostly ignored by physicians on the cardiac intensive care unit (CCU), not to mention treatment. Some studies about the effect of SAHS on AMI showed that SAHS would worsen the condition in patients with AMI. However, other studies in recent years showed that because of developed collateral vessels (11) and ischaemic preconditioning (12), SAHS may play a protective role for the heart from AMI. This was also supported by reported cases (13). So, there is no consensus on the issue.
To study those patients undergoing emergency pPCI, there are three reasons. One is to keep the states of the illnesses approximate, so we can have meaningful comparison. Another is that we can know the condition of patients’ coronary artery clearly. Last but not the least, there are no researches on this issue. The purpose of this survey is to find out the prevalence of SAHS among those patients and determine whether the chest onset time and the degrees of coronary stenosis and biomarkers of patients with AMI and SAHS change.
Methods
This was a prospective study. Between September 1 and December 31, 2015, patients with AMI undergoing emergency primary PCIs were all observed in the Cardiac Intensive Care Unit of Dongguan Kanghua Hospital. The emergency pPCI should be performed within 24 hours of hospitalisation. According to the guidelines for the treatment of AMI in 2004 made by the American Heart Association/American Heart Association (ACC/AHA) (14), AMI was diagnosed for every patient by at least two cardiologists. Informed consent was obtained from all individual participants included in the study.
Exclude criteria for patients: (I) accompanied by severe hepatic or renal inadequacy, pulmonary diseases (asthma, chronic obstructive pulmonary disease, diffuse pulmonary disease), or other cardiac diseases (valvular disease, congenital heart disease, cardiomyopathy) (2); (II) accompanied by neuromuscular diseases; (III) treated by oxygen therapy or CPAP within one month; (IV) taking hypnagogue or central stimulants or withdrawal within 1 week; (V) long severe alcohol abuse.
All eligible patients drew blood to test the values of the sensitivity of serum troponin T (hs-TnT), creatine kinase isoenzyme MB (CK-MB), pro-brain-type natriuretic peptide (pro-BNP) before the pPCI, four hours after the pPCI and the day after the pPCI. And then we chose the maximum value to analyze. Hs-TnT and pro-BNP were measured by electrochemiluminescence technique, and the normal ranges of their values are 0–14 and 0–70.7 pg/mL respectively. Immunosuppress was used to perform the level of CK-MB, and its normal range is 7–25 U/L. Echocardiogram was carried out the day after pPCI and left ventricular ejection fraction (LVEF) was measured.
We used the Gensini score to assess the severity of coronary artery damage. After the pPCI, we calculated the Gensini score of every patient as follows: 1 point for ≤25% narrowing, 2 points for 26% to 50% narrowing, 4 points for 51% to 75% narrowing, 8 points for 76% to 90% narrowing, 16 points for 91% to 99% narrowing, and 32 points for total occlusion. Each lesion score is multiplied by a factor that takes into account the importance of the lesion’s position in the coronary circulation (5 for the left main coronary artery, 2.5 for the proximal segment of the left anterior descending coronary artery, 2.5 for the proximal segment of the circumflex artery, 1.5 for the mid-segment of the left anterior descending coronary artery, 1.0 for the right coronary artery, the distal segment of the left anterior descending coronary artery, the posterolateral artery, and the obtuse marginal artery, and 0.5 for other segments). Finally, the Gensini score was calculated by summation of the individual coronary segment scores (15).
The grades of collateral vessels were classified by semi-quantitation (16): 0= none; 1= filling of side branches of the artery to be dilated via collateral channels without visualization of the epicardial segment; 2= partial filling of the epicardial segment via collateral channels; 3= complete filling of the epicardial segment of the artery being dilated via collateral channels.
Sleep studies of these patients were performed using a portable diagnostic device (LS-100, produced by Beijing Futian Electronic Medical Instrument Co., Ltd, Beijing, China). The parameters measured included nasal airflow (nasal cannula), pulse oximetry, snoring episodes (derived from the integrated pressure transducer) and pulse. Nurses in the CCU were trained before the study began. Two to seven days after the pPCI, nurses implemented the device when arterial oxygen saturation (SaO2) of patients ≥95% without oxygen inhalation. Nurses recorded the time that the patients went to sleep and went up. Seven hours were needed for night-time observation. When this failed to occur, the test was repeated. One reviewing physician interpreted the polysomnographic recordings. The definitions of apnoea and hypopnoea were based on the American Academy of Sleep Medicine criteria that were valid at the time of screening (2007/2008) (17). An apnoea hypopnoea index (AHI) was used for the diagnosis of SAHS and for classification: SAHS was diagnosed when AHI ≥5/h and was defined as mild for AHI ≥5/h and <15/h, moderate for AHI≥15/h and <30/h and severe for AHI ≥30/h.
Statistical analyses
Statistical analyses were conducted with the SPSS software package (version 19.0, SPSS, IBM). Quantitative dates (e.g., the age and BMI) were presented as the mean ± standard deviation (SD) and were compared by one-way ANOVA. The chi-square test compared categorical variables (e.g., smoker, diabetes, hypertension, arrhythmia, hyperlipidaemia). The frequency distribution of the onset of chest pain during the three-time intervals of the day was compared between patients with and without SAHS using the chi-square test. The AHIs of patients during the three intervals were expressed as medians (with interquartile ranges) and were compared using the Kruskal-Wallis test. A t-test was applied for comparisons between patients with and without SAHS. The relationship between AHI and several biomarkers, Gensini score, collateral vessels were analysed by correlation. For all tests, the P<0.05 was considered statistically significant.
Results
Prevalence of SAHS
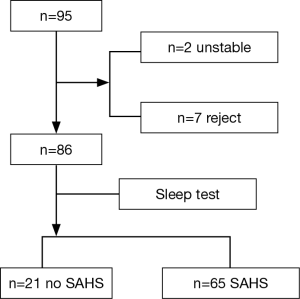
From September 1 to December 31, 2015, 95 patients were observed. Among those patients, two patients, both of whom were females aged older than 70 years, could not finish the sleep test because of their poor condition. Seven patients, all of whom were men, refused the sleep test. Finally, 86 patients completed the sleep test (Figure 1). Among those who finished the sleep test, 21 patients had no SAHS, and 65 patients had SAHS (AHI ≥5/h), representing an SAHS prevalence of 75.58% among patients with AMI undergoing emergency pPCI. Forty patients had a moderate-serious SAHS, representing 46.51% of patients, which requires further more attention.
Patient characteristics
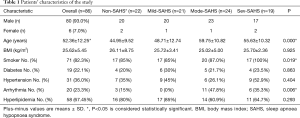
Patients’ characteristics in the study are described in Table 1.
Full table
From Table 1, we can see that, in all patients, 80 patients were men (93.0%), and 60 patients among them had SAHS (75%), while 6 patients were females (7.0%) and 4 patients among them had SAHS (66.7%). The average age of all patients in the study was 52.36±12.15 years, and the patients in the moderate-serious SAHS group was much older than the other groups. There were 71 patients (82.3%) who were smokers, and the moderate-serious SAHS group was more than the other groups. There were 20 patients (23.3%) who got arrhythmia, and the moderate-serious SAHS group was more stricken. Therefore, according to the statistical analysis, there were significant differences in average ages, smoking and arrhythmia (P<0.05) among the four groups (non-SAHS, mild-SAHS, moderate-SAHS and serious-SAHS). No significant differences were observed regarding BMI (body mass index P=0.925), diabetes (P=0.863), hypertension (P=0.404) and hyperlipidaemia (P=0.293).
Distribution of AHI throughout the day
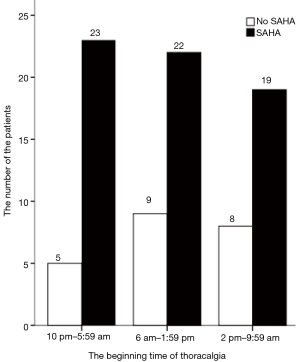
The frequency distribution of patients diagnosed with AMI with and without SAHS during the three eight-hour intervals of the day is shown in Figure 2. Patients with AMI and SAHS were much more frequent than those without SAHS during each interval (P<0.001). And from 10 pm to 5:59 am, the patients with AMI and SAHS were 23 which was slightly higher than those observed in 6 am to 1:59 pm and 2 pm to 9:59 pm (P=0.521), but this result was not statistically significant.
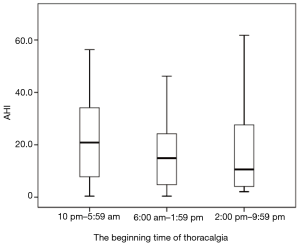
In Figure 3, we show the medians of AHI during the three intervals at the onset of chest pain. Between 10 pm–5:59 am, the median of AHI (20.85) was higher than during the other two intervals (14.9, 10.6). However, there were no significant differences among these three intervals (P=0.565).
Comparisons of biomarkers, LVEF, coronary artery Gensini scores and collateral vessels between patients with and without SAHS (Table 2). Relationships of AHI with biomarkers, LVEF, Gensini score and collateral vessels (Table 3)
From Table 2, we can see that the average Gensini score of patients with SAHS was 55.11±25.2, which was higher than those without SAHS which was 34.4±14.4. The average pro-BNP of patients with SAHS was 1,361.0±1,688.2 pg/mL, which was also higher than those without SAHS which was 526.5±805.0 pg/mL. And these two results had significantly statistical differences (P<0.05), while there were no statistical differences between the patients with and without SAHS in hs-TnT (P=0.826), CK-MB (P=0.591), LVEF (P=0.838), collateral vessels (P=0.702).
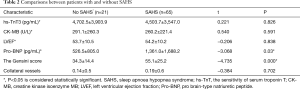
Full table
From Table 3, we see that there were positive correlations between AHI and Gensini scores (r=0.490, P<0.05) and pro-BNP (r=0.338, P<0.05) (Table 3), while neutral between AHI and hs-TnT (r=−0.013, P=0.902), CK-MB (r=−0.070, P=0.522), LVEF (r=0.007, P=0.948), collateral vessels (r=0.077, P=0.483).
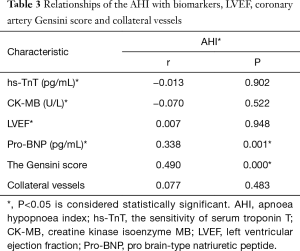
Full table
Discussion
SAHS is being studied not only in respiratory system but also in other fields, such as cardiovascular disease (CVD) and endocrinology. As we know, the influence of SAHS on CVD is of primary importance, especially regarding hypertension and coronary heart disease. Some published reports showed that SAHS with coronary heart disease (18,19) could worsen patient conditions. However, up to date, there have been no reports about the effect of SAHS in patients with AMI undergoing emergency pPCI, including the prevalence and the influence. These patients have a higher mortality rate than other patients with coronary heart disease. They also need methadone maintenance treatment after operation, which is a heavy burden for families and society. Therefore, we chose these patients to study.
In our participants, the ratio of male to female is 13.3:1, which is much higher than that in AMI (3:1–5:1) (20) and that in SAHS (2:1) (21). The particular population may be the major reason to cause this bias. Most of the participants’ ages are over 40 years, which fits the feature of AMI and SAHS. Also, the index of smoking, Arrhythmia and age show differences in these four teams. In our study, the prevalence of SAHS in patients with AMI undergoing emergency pPCI is 75.58%. Ben Ahmend et al. (22) reported a 79% prevalence of OSAHS with AMI for 120 people in France. Ludka et al. (10) reported a 65.7% prevalence in Brno, Czech Republic. In comparison with the prevalence of SAHS in CAD (approximately 60%), that in AMI undergoing emergency pPCI is much higher. Central sleep apnea may occur more often, which can increase the prevalence of SAHS.
Some people may think that the onset of AMI with SAHS should occur at midnight while people are sleeping. The result of our studies did not support the hypothesis. In our study, neither the number of SAHS nor the median of AHI in midnight wasn’t significantly higher than the other two periods We reviewed the related literatures and found that they had different conclusions on this issue. Some supported our result, such as Ludka’s (10) research while the others had the opposite opinion, for example, Kuniyoshi et al. (23) reported that there was a different diurnal variation among patients with AMI with or without SAHS. and Zhang et al. (2) reported that myocardial infarction mainly occurred between 10 pm and 6 am among patients with OSAHS.
In this study, we found that between SAHS and non-SAHS patients, there are significant differences in Gensini score and pro-BNP. Projective relationships between AHI and Gensini score and pro-BNP exist too. Kong et al. (24) reported that AHI was the most significant independent determinant of Gensini score among the coronary risk factors, which supported the result of our study. And Lu et al. (25) represented a positive correlation between AHI and Gensini score among patients with CAD. As we know, cardiologists place a high value on the condition of the coronary arteries, which greatly determine the severity and the prognosis of their patients. The Gensini score is the gold standard for judgement of the position, number and stenosis of blood vessels caused by coronary atherosclerosis (26). Therefore, the correlation revealed in our study is very important for cardiologists to predict the preoperative condition of the coronary arteries. If patients did not have preoperative sleep studies, the characteristics and the sleep descriptions of the patients would be references. Pro-BNP is increased in heart failure. Similarly, Takama et al. (27) reported that among OSAS patients, pro-BNP levels were significantly greater than among other patients. Because of the positive correlation between AHI and Gensini score and pro-BNP, patients with AMI undergoing emergency pPCI may benefit from treatment of SAHS. Losing weight, quitting alcohol and tobacco, side-lying position and other methods to change the bad habits of living are the basic treatments of SAHS, which could bring benefits to patients. Additionally, CPAP (28,29) may be helpful for patients with AMI and moderate-serious SAHS, but we should pay more attention to the problems of how to and when to use it appropriately.
There was no significant difference or correlation between Hs-TnT, CK-MB, LVEF and collateral vessels. Cifci et al. (30) also concluded that OSAS did not induce myocardial damage enough to increase CK, CK-MB and troponin I. Some reports (11,31) showed that patients with SAHS could have more collateral vessels, which may lead to myocardial preconditioning. However, our study did not support this finding.
In our study, we used a portable diagnostic device for diagnosing SAHS. The portable diagnostic device can be used as a good screening tool (32-34) for SAHS patients. Polysomnography (PSG) is a rather complex modality not applicable for patients who recently had operative procedures. Liang et al. (35) completed PSG and the portable monitor device testing simultaneously for 111 people with potential OSAS. They reached the conclusion that the portable diagnostic device had satisfactory sensitivity and specificity for diagnosing and judging the severity of OSAS. Takama et al. (27) reported that the portable device was effective in diagnosing SAHS in patients with CVD. It is also being used widely in our clinical work. So, we chose the portable device to study.
There are some limitations to this research. First, central SAHS and obstructive SAHS cannot be distinguished. Second, the samples are not enough and more patients should be observed and tested.
Conclusions
Our study reveals that SAHS has a high prevalence among patients with AMI undergoing emergency pPCI, which means whether a routine screening for SAHS should be performed to those patients with AMI, just like screening for hypertension and hyperlipidemia. Regarding the day-night pattern, there is no significant difference between AMI patients with or without SAHS. Furthermore, there are positive relationships between AHI and the coronary artery Gensini score and pro-BNP. This research presents clinicians with certain emerging implications. We may use CPAP with these patients for future research.
Acknowledgements
Thanks very much for all help and support of doctors and nurses in the Cardiac Intensive Care Unit in Dongguan Kanghua Hospital.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethics Statement: The study was approved by ethics committee of Dongguan Kanghua Hospital (No. 2015055) and written informed consent was obtained from all individual participants included in the study.
References
- Kato M, Adachi T, Koshino Y, et al. Obstructive sleep apnea and cardiovascular disease. Circ J 2009;73:1363-70. [Crossref] [PubMed]
- Zhang W, Sun Y, Li T, et al. The effect of obstructive sleep apnea-hypopnea syndrome on acute myocardial infarction. Bratisl Lek Listy 2012;113:565-8. [PubMed]
- Gonzaga C, Bertolami A, Bertolami M, et al. Obstructive sleep apnea, hypertension and cardiovascular diseases. J Hum Hypertens 2015;29:705-12. [Crossref] [PubMed]
- Li F, Huang H, Song L, et al. Effects of Obstructive Sleep Apnea Hypopnea Syndrome on Blood Pressure and C-Reactive Protein in Male Hypertension Patients. J Clin Med Res 2016;8:220-4. [Crossref] [PubMed]
- Cintra FD, Leite RP, Storti LJ, et al. Sleep Apnea and Nocturnal Cardiac Arrhythmia: A Populational Study. Arq Bras Cardiol 2014;103:368-74. [PubMed]
- Raghuram A, Clay R, Kumbam A, et al. A systematic review of the association between obstructive sleep apnea and ventricular arrhythmias. J Clin Sleep Med 2014;10:1155-60. [PubMed]
- Floras JS. Obstructive sleep apnea syndrome, continuous positive airway pressure and treatment of hypertension. Eur J Pharmacol 2015;763:28-37. [Crossref] [PubMed]
- Walia H, Strohl KP, Mehra R. Effect of continuous positive airway pressure on an atrial arrhythmia in a patient with mild obstructive sleep apnea. J Clin Sleep Med 2011;7:397-8. [PubMed]
- Liu L, Cao Q, Guo Z, et al. Continuous Positive Airway Pressure in Patients With Obstructive Sleep Apnea and Resistant Hypertension: A Meta-Analysis of Randomized Controlled Trials. J Clin Hypertens (Greenwich) 2016;18:153-8. [Crossref] [PubMed]
- Ludka O, Stepanova R, Vyskocilova M, et al. Sleep apnea prevalence in acute myocardial infarction--the Sleep Apnea in Post-acute Myocardial Infarction Patients (SAPAMI) Study. Int J Cardiol 2014;176:13-9. [Crossref] [PubMed]
- Ben Ahmed H, Boussaid H, Longo S, et al. Impact of obstructive sleep apnea in recruitment of coronary collaterality during inaugural acute myocardial infarction. Ann Cardiol Angeiol (Paris) 2015;64:273-8. [Crossref] [PubMed]
- Shah N, Redline S, Yaggi HK, et al. Obstructive sleep apnea and acute myocardial infarction severity: ischemic preconditioning? Sleep Breath 2013;17:819-26. [Crossref] [PubMed]
- Moraes RC, Mangili LC, Benvenuti LA. Case 1 /2011--seventy-four-year-old female patient with sudden apnea and acute cholecystitis, five days after acute myocardial infarction without critical coronary lesions. Arq Bras Cardiol 2011;96:e35-41. [PubMed]
- Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction; A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction). J Am Coll Cardiol 2004;44:E1-E211. [Crossref] [PubMed]
- Avci A, Fidan S, Tabakci MM, et al. Association between the Gensini Score and Carotid Artery Stenosis. Korean Circ J 2016;46:639-45. [Crossref] [PubMed]
- Rentrop KP, Cohen M, Blanke H, et al. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol 1985;5:587-92. [Crossref] [PubMed]
- Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012;8:597-619. [PubMed]
- Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation 2010;122:352-60. [Crossref] [PubMed]
- Martinez D, Klein C, Rahmeier L, et al. Sleep apnea is a stronger predictor for coronary heart disease than traditional risk factors. Sleep Breath 2012;16:695-701. [Crossref] [PubMed]
- Coppieters Y, Collart P, Leveque A. Gender differences in acute myocardial infarction, twenty-five years registration. Int J Cardiol 2012;160:127-32. [Crossref] [PubMed]
- Wahner-Roedler DL, Olson EJ, Narayanan S, et al. Gender-specific differences in a patient population with obstructive sleep apnea-hypopnea syndrome. Gend Med 2007;4:329-38. [Crossref] [PubMed]
- Ben Ahmed H, Boussaid H, Hamdi I, et al. Prevalence and predictors of obstructive sleep apnea in patients admitted for acute myocardial infarction. Ann Cardiol Angeiol (Paris) 2014;63:65-70. [Crossref] [PubMed]
- Kuniyoshi FH, Garcia-Touchard A, Gami AS, et al. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol 2008;52:343-6. [Crossref] [PubMed]
- Kong L, Guo XH. The relation between obstructive sleep apnea hypopnea syndrome and the severity of coronary atherosclerosis in coronary artery disease. Zhonghua Nei Ke Za Zhi 2009;48:638-42. [PubMed]
- Lu G, Xu ZW, Liu JN, et al. A study on the association of obstructive sleep apnea hypopnea syndrome with coronary atherosclerosis and coronary heart disease. Zhonghua Jie He He Hu Xi Za Zhi 2007;30:178-81. [PubMed]
- Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 1983;51:606. [Crossref] [PubMed]
- Takama N, Kurabayashi M. Effectiveness of a portable device and the need for treatment of mild-to-moderate obstructive sleep-disordered breathing in patients with cardiovascular disease. J Cardiol 2010;56:73-8. [Crossref] [PubMed]
- Qi Y, Cai M, Zhang HM, et al. Impact of long-term continuous positive airway pressure treatment for patients with obstructive sleep apnea syndrome. Zhonghua Xin Xue Guan Bing Za Zhi 2016;44:144-9. [PubMed]
- Msaad S, Marrakchi R, Grati M, et al. How does serum brain natriuretic peptide level change under nasal continuous positive airway pressure in obstructive sleep apnea-hypopnea syndrome? Libyan J Med 2016;11:31673. [Crossref]
- Cifçi N, Uyar M, Elbek O, et al. Impact of CPAP treatment on cardiac biomarkers and pro-BNP in obstructive sleep apnea syndrome. Sleep Breath 2010;14:241-4. [Crossref] [PubMed]
- Steiner S, Schueller PO, Schulze V, et al. Occurrence of coronary collateral vessels in patients with sleep apnea and total coronary occlusion. Chest 2010;137:516-20. [Crossref] [PubMed]
- Danzi-Soares NJ, Genta PR, Nerbass FB, et al. Obstructive sleep apnea is common among patients referred for coronary artery bypass grafting and can be diagnosed by portable monitoring. Coron Artery Dis 2012;23:31-8. [Crossref] [PubMed]
- Lesser DJ, Haddad GG, Bush RA, et al. The utility of a portable recording device for screening of obstructive sleep apnea in obese adolescents. J Clin Sleep Med 2012;8:271-7. [PubMed]
- Ferré A, Sampol G, Jurado MJ, et al. Neurophysiological two-channel polysomnographic device in the diagnosis of sleep apnea. J Clin Sleep Med 2012;8:163-8. [PubMed]
- Liang S, Chen Z, Huang J, et al. Diagnostic value of portable monitor device in patients with potential obstructive sleep apnea hypopnea syndrome. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2016;51:209-11. [PubMed]