New generation bioresorbable scaffold technologies: an update on novel devices and clinical results
Introduction
Bioresorbable scaffolds (BRS) represent the fourth evolution in myocardial revascularization therapies with extensive technological development and advancements. Indeed, the idea of a biodegradable scaffold that can restore anatomical and physiological characteristics of the treated vessel once it has provided enough support is potentially a good long-term alternative to address some of the limitations of drug-eluting stents (DES). As of today, over 20 companies are working on new devices or on refining existing scaffolds.
Future BRS technologies and challenges
As of April 2017, five BRSs received CE mark approval for use in Europe, four of them polymeric scaffolds and one metallic: Absorb BVS (Abbott Vascular, Santa Clara, CA, USA), DESolve (Elixir Medical Corporation, Sunnyvale, California, USA), Fantom (REVA Medical, Inc., San Diego, CA, USA), ART (Terumo, Tokyo, Japan) and the magnesium based Magmaris (Biotronik, Berlin, Germany). The only device available outside Europe, with FDA (United State market) and PDMA (Japan market) approval, is Absorb BVS.
Beside the aforementioned available devices, we will focus on scaffolds that are not commercially available. Among them Falcon BVS (Abbott Vascular, Santa Clara, CA, USA), Fortitude, Aptitude and Magnitude (Amaranth Medical, Mountain View, USA), MeRes (Meril Life Science, Gujarat, India), MIRAGE (Manli Cardiology, Singapore), Renuvia (Boston Scientific, Marlborough, MA, USA), Xinsorb (Huaan Biotech, Upper Heyford, UK) and Firesorb (MicroPort, Shanghai, China) use poly-L lactide (PLLA) as main component. The core of IDEAL (Xenogenics Corp, Canton, MA, USA) is made of polylactide anhydride mixed with a polymer of salicylic acid and sebacic acid linker. The Unity Hybrid BRS (Qualimed, Winsen, Germany) uses a combination of metallic bone made of magnesium with a PLLA coating.
Challenging points of BRS development
The main challenge of BRS development is to find a way of fine tuning the mechanical properties between providing enough vessel support to prevent recoil in the first months after implantation and minimizing the resorption time in order to reduce possible late events (Table 1, Figure 1).

Full table
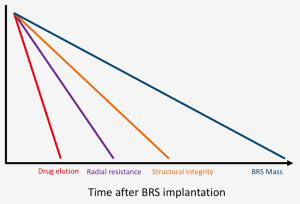
Strut thickness, width and design, together with the type of material utilized and the crimping method are all extremely important features to take into account. In particular, the tensile modulus (and the directly related radial strength) of bioresorbable materials is well inferior to the metallic alloys used for DES; this leads to increased strut thickness and width, and therefore higher scaffold footprint (defined as the percentage of vascular circumference occupied by struts). The increase in strut thickness and width has also direct repercussions on the endothelialization process and side branch occlusion and access. Indeed, thinner struts may result in better strut embedment and more rapid strut coverage, which could potentially prevent the negative effects of late malapposition such late scaffold intraluminal dismantling. Moreover, thicker struts result in increased crossing profile, thus making deliverability an issue (1-3).
A second crucial aspect is the limited over-expansion capability of first generation BRS. This may lead to an increased risk of strut fracture upon deployment, and therefore to late thrombotic events related to incomplete coverage of the fractured portions as well as the inability to fully correct malapposition. The urge to improve this feature should be addressed while maintaining radial strength (4).
Thirdly, a limitation of currently available BRSs is the low visibility: the polymeric structure makes the struts virtually radiolucent, while the radiopaque markers may be difficult to identify especially in calcific lesions or in obese patients.Current devices are also limited by the being crimped on a very compliant balloon in order to minimize crossing profile and to have better deliverability and scaffold retention; this hampered the possibility to implant BRS at high pressure, thus making postdilatation mandatory in order to achieve optimal results. Furthermore, the use of such compliant balloons may lead to insufficient crimping or to scaffold loss during the delivery.Another practical issue that may limit the diffusion of BRSs, is that some devices require special storage facilities, that add costs to the procedure and may not be available in every hospital.Finally, a paramount aspect of BRS implantation is the optimal duration and type of dual antiplatelet therapy (DAPT). Thicker and wider struts may lead to a higher risk of thrombosis and may take more time to be completely endothelialized. While current guidelines do not suggest prolonged DAPT, it may be considered in patients without high bleeding risk. This scenario may change with BRS with decreased strut thickness and shorter reabsorption time.
Future BRS overview
A vast amount of literature evaluated the performance of the most widely available BRS (Absorb BVS) both in pre-clinical and clinical settings. Large data are also available for Magmaris and DESolve. This review is intended to highlight technical characteristics and clinical outcomes of other BRSs.
REVA Medical (Fantom)
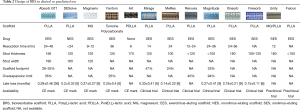
The main peculiarity of this sirolimus-eluting desaminotyrosine polycarbonate-based scaffold is that it contains iodine covalently bound to the polymer backbone. This feature makes it intrinsically radio-opaque (Table 2) throughout the whole degradation process. This feature may decrease the need for intravascular imaging modalities such as IVUS and OCT. Overall, less than 3% iodine of what is normally contained in 1 mL of contrast is present in each single device, and is then safely excreted.
Full table
With a strut thickness of 125 µm, the device showed good flexibility and deliverability, and maintained good radial strength. Moreover, the expandibility range of this platform is extremely wide: post-dilation is possible with a 0.75–1 mm excess depending on device size. Preclinical studies have shown restoration of vasomotor response at 12-month follow-up (5,6). Reabsorption time is about 36 months, longer than PLLA scaffolds, but more than 80% molecular weight loss takes place in the first 12 months.
A pilot clinical trial, FANTOM-I, enrolled seven patients to verify acute performance of the device (7). Safety and performance of the device were studied in the FANTOM II trial on 240 patients: major adverse cardiovascular events (MACE) rate at 6 months was 2% and late lumen loss (LLL) was 0.25±0.40 mm; scaffold thrombosis (ScT) was reported in one patient (0.4%) in which the target lesion was not fully covered with the scaffold. At 6-month IVUS follow-up, 98% of struts were covered (8). The 12-month outcomes showed a MACE rate of 4.2%, including two cardiac deaths, three target-vessel MIs, but no occurrences of late thrombosis. CE Mark approval for the Fantom scaffold was received in April 2017.
Terumo (Art)
The bare ART BRS is an amorphous polymer of PDLLA (Table 2). Complete bioresorption ends within 18 months, and structural integrity lasts 5 to 7 months. Preclinical studies reported no MACE and BMS-like acute recoil rates; IVUS at 9-month follow-up confirmed increased mean lumen area and external elastic lamina area (9,10). Safety and efficacy were then to be tested in the first in man trial Arterial Remodeling Transient Dismantling Vascular Angioplasty (ARTDIVA) (11). No myocardial infarction (MI) or stroke was observed, while 1 ischemia driven and 2 non-ischemia driven target lesion revascularizations (TLR) were registered (12). CE Mark was achieved in May 2015. No other clinical result is available to date.
Abbott (Falcon)
While over 150,000 patients have been treated with Absorb BVS, the company developed a newer generation device that is designed to maintain benefits of current BVS (restore vessel and improve clinical outcomes) and minimize the versatility gap that exists in comparison to metallic DES (13). The future Falcon BVS is rumored to have thinner struts (probably under 100 µm) and improved scaffold profile, in order to improve deliverability and acute performance. Furthermore, the deployment balloon will be less compliant as compared to the one used in the current generation, making implantation easier and safer. The everolimus-eluting scaffold will maintain PLLA structure but, thanks to broader size matrix, it will have reduced footprints on the vessel, thus improving healing process and reducing the risk of scaffold-related adverse events. The device was implanted only in animal models, and no clinical data are available.
Amaranth (Fortitude, Aptitude and Magnitude)
The Amaranth scaffold family is made of sirolimus-eluting BRSs with peculiar polymer production features (Table 2): in contraposition with the crystalline frame of other PLLA scaffolds, these scaffolds are made of an ultra high molecular weight resin with an amorphous structure, which increases its elongation at break with respect to typical polymer capabilities. Abbott BVS scaffold tube fabrication is by extrusion whereas the Amaranth BRSs are processed by solution casting. Radial force is comparable to metallic stents, and the miniaturization process from 150 to <100 µm did not seem to alter this aspect. The result is a device with elevated fracture toughness and over-expansion capabilities. Indeed, these scaffolds may resist up to 2.5 mm over nominal diameter that is 2.5 times higher over-expansion capabilities with respect to Absorb BVS. Moreover, the surface area of coverage is slightly lower than Absorb BVS (about 25% compared to 26–32%) (14).
A 4-year experience overall, and a 2-year human experience have successfully proven biocompatibility and biomechanical sustainability. Clinical programs progressed with the evolution of the scaffold: MEND I and II trial evaluated the bare 150 µm Fortitude performance at one site in Colombia. The 2-year results in terms of in-scaffold LLL were encouraging with a net gain of 0.3±0.2 mm. No scaffold restenosis or thrombosis was observed in the same timeframe (14).
The sirolimus-eluting version of the same scaffold was evaluated in the RENASCENT-I trial, a prospective, multi-center, single arm study that had promising data so far. Results at 9 months QCA showed an in-scaffold LLL of 0.27±0.41 mm and binary restenosis rate of 1.6%. Concerning safety endpoints at 9 months, the MI rate was 3.3% and ischemia-driven TLR was 1.6%, for a total target lesion failure (TLF) of 4.9%. One non-cardiovascular mortality occurred, while no ScT was observed (14).
No clinical data are available for the second and third generation of the device: the RENASCENT-II enrolled patients treated with the Aptitude sirolimus-eluting scaffold that has 115 µm struts. Nine months clinical and imaging results showed high clinical success (98.3%), a low MACE rate (3.4%), and no angiographic restenosis or ScT. Scaffold stability as assessed by optical coherence tomography was maintained at 9 months, as were a high level of strut coverage and low rate of malapposition. The RENASCENT-III trial will address outcomes of the sub-100 µm Magnitude scaffold (14).
Boston Scientific (Renuvia)
Renuvia (Table 2) is a 115 µm PLLA everolimus-eluting scaffold produced by Boston Scientific. Its unique scaffold architecture and delivery system are derived from the ones used in Synergy DES, and were developed in order to ensure good deliverability (15). Bench tests showed good radial strength and overexpansion capability. A first in man trial (RENUVIA FAST) is currently enrolling patients with simple lesions (16): early data supports the deliverability and trackability of the device.
Xenogenics (Ideal)
The core of IDEAL BRS is of polylactide anhydride mixed with a polymer of salicylic acid and sebacic acid; a salicylate coating controls elution of sirolimus, and it possibly adds an anti-inflammatory effect to the anti-proliferative action of the macrocyclic lactone (17). Unfortunately, a significant reduction in lumen area was observed due to unsatisfactory neointimal suppression in the first human trial in 2009. Thought to be related to the too rapid elution and the too low surface area dose of the antiproliferative drug (18), a new generation IDEAL BioStent device is now entering preclinical studies.
Meril (MeRes)
MeRes BRS is sirolimus-eluting and it has a backbone of PLLA coated by PDLLA for drug release control (Table 2). Peculiar features are the hybrid scaffold geometry that yields high radial strength, tri-axial radiopaque markers that facilitate the procedure and thin struts (100 µm) that allow also easy side branch access. The healing response in porcine models was promising, with low thrombogenicity and good biomechanical stability. MeRes-1 clinical trial enrolled 108 patients across medical centres in India (19). Reported 6-month LLL was 0.14±0.22 mm with no binary restenosis; no TLF or ScT occurred during follow-up. At 1-year computed tomography angiography demonstrated that all scaffolds were patent and with only mild mean percentage area stenosis.
Manli Cardiology (Mirage)
The Mirage bioresorbable micro-fibre scaffold is PLLA-based and sirolimus-eluting (Table 2). Strut thickness varies with scaffold diameter (125 µm in ≤3 mm, and 150 µm in ≥3.5 mm). The main feature is the helix coil design that allows good flexibility and low crossing profile; bioresorption time is 14 months.
A preclinical study on porcine models showed promising results; in particular, no in-scaffold restenosis was observed at 6-month follow-up, 99% of the struts were covered, and mean NIH thickness was 0.08±0.03 mm (20). Only one malapposed strut was evident at 6-month follow-up. At 6 months, LLL was 0.21±0.20 mm, while no in-scaffold and in-segment binary restenosis were present (20).
A prospective, multi-center, single blinded, clinical investigation versus Absorb BVS has performed and randomized 60 patients to treatment with Mirage or Absorb (21). The study was performed with both angiographic and OCT endpoints and showed that at 12 months, angiographic in-scaffold late loss was not statistically different between the Mirage and Absorb devices (0.37 and 0.23 mm respectively), although diameter stenosis on angiography and on optical coherence tomography was significantly higher with the Mirage than with the Absorb (31.8%±12.9% vs. 21.2%±9.9%). Anyway, these findings this did not translate into either angiographic binary restenosis or clinical outcomes: TLF was 17.2% in Mirage group and 14.8% in Absorb group, with only one ScT in the Mirage group.
HuaAn Biotechnology (Xinsorb)
This sirolimus-eluting scaffold made of PLLA has a strut thickness of 160 µm (Table 2). Preclinical studies comparing Xinsorb BRS and the Excel DES (JW Medical; Shandong, China) found no significant difference in restenosis rates at 6 months (22). The in-scaffold LLL at 1, 3, 12 and 18 months follow-up was 0.68±0.42, 0.77±0.48, 0.28±0.41 and 0.09±0.31 mm, respectively, with a significant increase of lumen area between the 3- and 18-month follow-up (22). LLL observed at 6-month follow-up in Xinsorb FIM trial was 0.18±0.21 mm with a subset of patients evaluated with IVUS and OCT with good results and only mild neointimal thickness (23).
MicroPort (Firesorb)
This sirolimus-eluting PLLA scaffold (Table 2) has thinner struts as compared to Absorb (100 µm for 2.5 mm device and 125 µm for larger devices) and lower drug dosage, in order to achieve a faster strut coverage and reabsorption (24). FUTURE-I early study enrolled 45 patients and showed low event rate: one MI and one revascularization, but no deaths or ScT at 1 year. Furthermore, 99% of struts were covered after 6 months and LLL were about 0.15±0.11 mm. A pivotal randomized controlled trial (FUTURE-II) will be initiated soon.
QualiMed (Unity)
This hybrid sirolimus-eluting scaffold combines a bioresorbable magnesium core with a bioresorbable polymer coating made of PLLA (Table 2). Strut thickness is about 160 µm; nevertheless, crossing profile is reduced as compared to Absorb BVS. Also overexpansion limits are wider, allowing a more forgiving implantation. Reabsorption process is similar to PLLA scaffolds, about 12 months. This device is already marketed for peripheral vessels, while no clinical data are available for coronary use (25).
Conclusions
Following the encouraging results of real-world implantation of the commercially available BRSs, there have been huge investments on developing biodegradable devices for the treatment coronary artery disease. Like all early generation devices, they showed some limitations, mainly related to the difficult balance between the reabsorption process and the need of mechanical vessel support: the ideal device must have low rates of early and late events (particularly ScT), LLL comparable to DES, be operator forgiving in terms of deliverability, visibility and of need of a dedicated implantation technique, easy to store. Many device companies are currently working on new BRSs, developing alternative materials, thinner struts, better drugs and scaffold architectures, in order to overcome these limitations, as shown in this review. While technological developments are needed in order to fill the gap with current metallic DESs, we believe that BRSs are one of the most relevant innovations in coronary interventions and may eventually lead to long term clinical benefits.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Latib has received speaking honoraria from Abbott Vascular and is a consultant from Medtronic & Amaranth Medical; the other authors have no conflicts of interest to declare.
References
- Foin N, Lee RD, Torii R, et al. Impact of stent strut design in metallic stents and biodegradable scaffolds. Int J Cardiol 2014;177:800-8. [Crossref] [PubMed]
- Waksman R, Pakala R. Biodegradable and bioabsorbable stents. Curr Pharm Des 2010;16:4041-51. [Crossref] [PubMed]
- Foin N, Torii R, Mattesini A, et al. Biodegradable vascular scaffold: is optimal expansion the key to minimising flow disturbances and risk of adverse events? EuroIntervention 2015;10:1139-42. [Crossref] [PubMed]
- Ormiston JA, De Vroey F, Serruys PW, et al. Bioresorbable polymeric vascular scaffolds: a cautionary tale. Circ Cardiovasc Interv 2011;4:535-8. [Crossref] [PubMed]
- Costa R. Planned and ongoing clinical trials: A status update from the Cardiovascular Research Center (CRC). New bioresorbable scaffolds programmes: REVA and MIRAGE. EuroPCR; France 2014. Available online: http://www.pcronline.com/Lectures/2014/New-bioresorbable-scaffolds-programmes-REVA-and-MIRAGE
- Abizaid A. Other bioresorbable scaffold materials tyrosine polycarbonate. EuroPCR; France 2014. Available online: http://www.pcronline.com/Lectures/2014/Other-polymer-based-scaffolds
- Abizaid A. FantomTM Sirolimus-eluting bioresorbable scaffold. EuroPCR; France 2015. Available online: http://www.pcronline.com/Lectures/2015/A-bioresorbable-sirolimus-eluting-scaffold
- Stone GW. FANTOM: A radio-opaque desaminotyrosine polycarbonate-based scaffold. Transcatheter Cardiovasc Therapeutics. Washington, DC, USA; October 31st 2016.
- Lafont A, Durand E. ART: concept of a bioresorbable stent without drug elution. EuroIntervention 2009;5 Suppl F:F83-7.
- Durand E, Lemitre M, Couty L, et al. Adjusting a polymer formulation for an optimal bioresorbable stent: a 6-month follow-up study. EuroIntervention 2012;8:242-9. [Crossref] [PubMed]
- Fajadet J. The ART, stent: design and early first-in-man experiences. Transcatheter Cardiovasc Therapeutics. Miami Beach, FL, USA; October 23rd 2012.
- Lafont A. ARTDIVA. BRS 2014. Boston, MA, USA; July 6th 2014.
- Rapoza R. Next generation Absorb: a workhorse BVS. Transcatheter Cardiovasc Therapeutics. Washington, DC, USA; October 31st 2016.
- Latib A. Fortitude, aptitude and magnitude: Other thin-strut novel PLLA-based scaffolds. Bioresorbable vascular scaffolds at JIM 2017: a clinical workshop. JIM, Milan, Italy; February 14th 2017.
- Dawkins K. The RENUVIA thins struts BRS platform. JIM, Milan, Italy; February 14th 2017.
- Meredith IT. RENUVIA FAST First Human clinical trial. Transcatheter Cardiovasc Therapeutics. Washington, DC, USA; October 31st 2016.
- Jabara R, Chronos N, Robinson K. Novel bioabsorbable salicylate-based polymer as a drug-eluting stent coating. Catheter Cardiovasc Interv 2008;72:186-94. [Crossref] [PubMed]
- Jabara R, Pendyala L, Geva S, et al. Novel fully bioabsorbable salicylate-based sirolimus-eluting stent. EuroIntervention 2009;5 Suppl F:F58-64.
- Granada J. Fully Bioresorbable PLLA-Based Sirolimus-Eluting MeRes 100 Scaffold (Meril Life Science). EuroPCR; France 2015. Available online: http://www.pcronline.com/Lectures/2015/Fully-bioresorbable-PLLA-based-sirolimus-eluting-MeRes-100-scaffold-Meril-Life-Science-India
- Santoso T. The Mirage Bioresorbable Microfiber Scaffold (BRMS) Manli Cardiology. Transcatheter Cardiovasc Therapeutics. Washington, DC, USA; September 16th 2014.
- Tenekecioglu E, Serruys PW, Onuma Y, et al. Randomized Comparison of Absorb Bioresorbable Vascular Scaffold and Mirage Microfiber Sirolimus-Eluting Scaffold Using Multimodality Imaging. JACC Cardiovasc Interv 2017;10:1115-30. [Crossref] [PubMed]
- Wu Y, Shen L, Yao Z, et al. Long-term Angiographic and Optical Coherence Tomography Follow-up of XINSORB Scaffold in Porcine Coronary Model. Transcatheter Cardiovasc Therapeutics 2014. Washington, DC, USA; September 13-17th 2014.
- Ge J. BRS Under Development III – The XINSORB BRS. Transcatheter Cardiovasc Therapeutics 2014. Washington, DC, USA; September 16th 2014.
- Xu B. Firesorb PLLA-based sirolimus-eluting scaffold. Transcatheter Cardiovasc Therapeutics. Washington, DC. USA; October 31st 2016.
- Latib A. Qualimed UNITY Hybrid BRS. JIM, Milan, Italy; 2016.


