Unique distribution of programmed death ligand 1 (PD-L1) expression in East Asian non-small cell lung cancer
Introduction
With advances in the investigation of mechanisms on immune recognition and immune escape, and development of immune checkpoint blockage agents, the treatment of non-small cell lung cancer (NSCLC) has entered into a new era of immunotherapy. Recent years, multiple clinical trials were conducted to assess the efficacy of immunotherapy antibodies, as well as the predictive value of PD-L1 expression from different antibody manufacturers. However, controversy still remains that there is no specific relationship between positive PD-L1 expression and response rate in patients receiving immunotherapy (1-4). As patients who cannot benefit from immunotherapy may suffer quick progression after receiving immunotherapy (5), it is urgent to find a biomarker which could effectively predict the efficacy of anti-PD-1/PD-L1 treatment. The serial of KEYNOTE clinical trials showed high response rates of pembrolizumab ranged from 44.6% to 45.2% among patients with NSCLC (6-8). And the efficacy of this anti-PD-1 agent was closely related to the expression of PD-L1 with a tumor proportion score (TPS) at least 50% which was detected by PD-L1 IHC 22C3 pharmDx Assay. Based on this encouraging data, using antibody specific PD-L1 expression in tumors seems optimal approach to screen eligible patients for immunotherapy to date.
Although PD-L1 IHC 22C3 pharmDx Assay was quite effective and reliable (9), and was till now the only companion diagnostic approved by the FDA as an aid in identifying patients with NSCLC for treatment with KEYTRUDA® (pembrolizumab), cost of this test could not be ignored. In a cost-effectiveness perspective, it was unreasonable to perform PD-L1 expression testing for all NSCLC patients.
On the other hand, current data of PD-L1 expression on NSCLC was mainly based on Western populations, situation on Asian populations remained unclear. Spectrum of PD-L1 expression and association between PD-L1 expression and clinicopathological variables of Asian patients with NSCLC were to be revealed. Therefore, we hope to identify some clinical and pathological features which could help narrow done the qualified patients for immunotherapy and facilitate the choice of treatment and enrollment of clinical trials.
In this study, we carried out an immunohistochemistry (IHC) investigation of PD-L1 expression in surgically resected lung squamous cell carcinomas (SCC) and adenocarcinomas using PD-L1 IHC 22C3 antibody, and correlated PD-L1 expression level with clinicopathological and molecular parameters, including pathological subtypes, age, gender, smoking history, tumor size and common driver mutations. Meanwhile, the prognostic value of PD-L1 expression was also assessed.
Methods
Specimen collection
Lung cancer samples were collected from patients who underwent surgical resection with curative intent in our institution from September 2009 to March 2013. To validate the IHC procedure, a cohort of newly resected lung cancer samples was also collected in 2017. Eligible patients were required to have sufficient tissue for immunohistochemical staining and comprehensive mutational analyses. Patients who received neoadjuvant chemotherapy, had a history of malignant tumors, as well as without integrated clinicopathological and survival data were excluded. All cases were reviewed by two pathologists for confirmation of tumor histology and tumor content. This study was approved by the institutional review board of the Shanghai Cancer Center, Fudan University, Shanghai, China. All patients underwent surgery and provided written informed consent.
IHC and interpretation
Tissue specimen preparation and IHC
All specimens used in this IHC procedure were formalin fixed paraffin-embedded (FFPE). Sections were cut at 4 µm thickness, dried for 1 hour at 60 °C and stored in dark at 4°C. IHC staining procedure was performed using the Dako Autostainer Link 48 platform and an adjusted automated staining process from validated protocol for the PD-L1 IHC 22C3 (9). As only Monoclonal Mouse Anti-Human PD-L1 antibody (Clone 22C3, Dako) was purchased, other required agents were replaced by a validated kit from Dako company [EnVisonTM FLEX +, Mouse, High pH, (Link), Code K8002]. Deparaffinization, rehydration, and target retrieval was performed in the PT Link (Dako PT100) using a 3-in-1 procedure. After incubation with the monoclonal mouse anti-human PD-L1 antibody, clone 22C3, specimens were incubated with anti-mouse linker antibody specific to the host species of the primary antibody, and then were incubated with a ready-to-use visualization reagent consisting of secondary antibody molecules and horseradish peroxidase molecules coupled to a dextran polymer backbone. The specimens were then counterstained with hematoxylin and cover-slipped. Results were interpreted using a light microscope by two pathologists (Yuan Li and Xiaoyan Zhou).
TPS
For determination of PD-L1 protein expression, positivity was defined as complete circumferential or partial cell membrane staining of viable tumor cells with 1+ to 3+ intensity. Tumor associated immune cells were excluded from PD-L1 scoring. Cytoplasmic staining, was excluded from the scoring. Scoring was recorded as percentage of PD-L1-positive tumor cells over total tumor cells in the denominator (TPS). NSCLC specimens stained with the negative control reagent must have 0 specific membrane staining and ≤1+ intensity nonspecific (nonmembrane) staining.
Mutational analysis
Apart from PPFE samples, surgical resected lung cancer specimens were routinely collected and stored in liquid nitrogen until use in our center, which has been well described in our previous studies. Genomic DNA and RNA were extracted as per standard protocols (RNeasy Mini Kit, and QiAamp DNA Mini Kit, Qiagen, Hilden, Germany). Total RNA samples were reverse transcribed into single-stranded cDNA using RevertAid First Strand cDNA Synthesis Kit (Fermentas, St Leon-Rot, Germany). Either genomic DNA or cDNA were used for polymerase chain reaction (PCR) amplification and sequencing. EGFR (exons 18-22), HER2 (exons 18 to 21), KRAS (exons 2 to 3), and BRAF (exons 11 to 15) were PCR amplified using cDNA for further sequencing. FISH assay and real-time PCR were simultaneously used in detecting ALK, ROS1 and RET translocations. Samples without EGFR, KRAS, HER2, BRAF mutations and ALK/ROS1/RET translocations were defined as “pan-negative”.
Statistical analysis
Pearson’s chi-squared test or Fisher’s exact test was used to assess correlations between different PD-L1 expression level and clinicopathologic variables as well as mutational status. Kaplan–Meier method was used to draw the survival curves. Relapse-free and overall survival of patients with TPS ≥50% or <50% immunostaining was compared using the log-rank test. The statistical analyses were done using SPSS 16.0 (IBM Corporation, Armonk, NY, USA) and GraphPad Prism 6 (GraphPadCorp, San Diego, CA, USA). The two-sided significance level was set at P<0.05.
Results
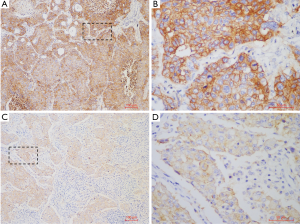
A total of 329 NSCLC cases including 108 SCCs and 221 lung adenocarcinomas (LUADs) were screened for PD-L1 expression, among which, 10 SCC samples were collected in 2017 which are lack of sufficient follow-up time were only used in PD-L1 expression analysis. Totally, 16 (4.9%) cases were found with PD-L1 high (TPS ≥50%) expression, 30 (9.1%) cases with PD-L1 low (TPS <50%) expression, and 283 (86%) cases with PD-L1 negative staining. In subgroup analysis, positive PD-L1 expression was found in 37 lung SCCs (37/108, 34.3%), including 15 cases with TPS ≥50% (15/108, 13.9%) and 22 cases with TPS <50% (22/108, 20.4%). In LUAD cohort, 9 cases were found PD-L1 expression positive (9/221, 4.1%), including 1 case with TPS ≥50% (1/221, 0.5%) and 8 cases with TPS <50% (8/221, 3.9%) (Figure 1). The representative photos of PD-L1 high expression in the SSCs (Figure 2A,B) and LUADs (Figure 2C,D) were shown in Figure 2.
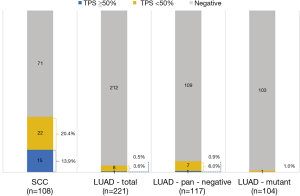
Of those patients with PD-L1 TPS ≥50%, 15 (15/16, 93.8%) were male and 14 (14/16, 87.5%) were current or ever smokers. Of those samples with PD-L1 TPS ≥50%, 15 (15/16, 93.8%) were SCCs, 7 (7/16, 43.8%) were with tumor size less than or equal to 3 cm, 9 (9/16, 46.2%) were N0 status and 8 (8/16, 50%) were stage I (Figure 1, Table 1). Notably, in the LUAD subgroup, 1 sample with PD-L1 TPS ≥50% and 7 samples with PD-L1 TPS <50% were found in the pan-negative LUAD group, and only 1 sample with PD-L1 TPS <50% was found in the LUADs with EGFR mutation (Figure 1).
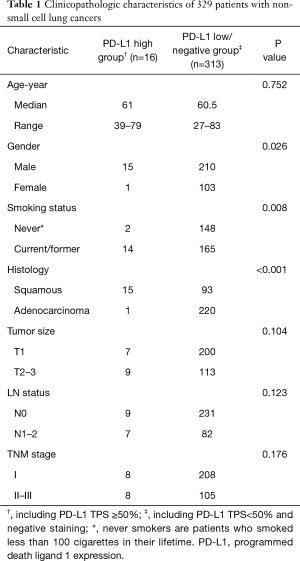
Full table
Comparison of clinicopathologic characteristics was performed between PD-L1 high expression group (TPS ≥50%) and PD-L1 low/negative group. The results showed that high PD-L1 expression was significantly associated with male sex (p=0.026), current/ever smoking history (P=0.008) and SCC subtype (P<0.001) (Table 1).
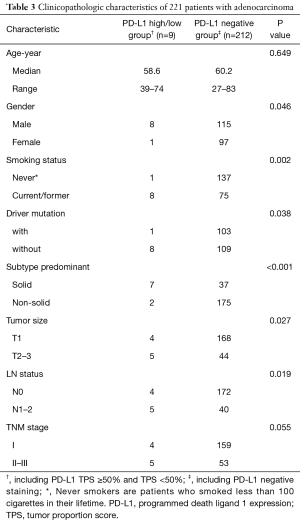
Furthermore, no significant difference was identified between PD-L1 high (TPS ≥50%) and low/negative expression groups in the SCCs subgroups (Table 2). As only 1 sample with PD-L1 high expression (TPS ≥50%) was found in LUAD group, comparison of clinicopathologic characteristics was performed between PD-L1 positive and negative groups. We found that positive PD-L1 expression (including TPS ≥50% and TPS <50%) in LUAD cohort was significantly associated with male sex (P=0.046), current/ever smoking history (P=0.002), mutation pan-negative status (P=0.038), solid-predominant subtype (P<0.001), large tumor size (P=0.027) and lymph node metastasis (P=0.019) (Table 3).
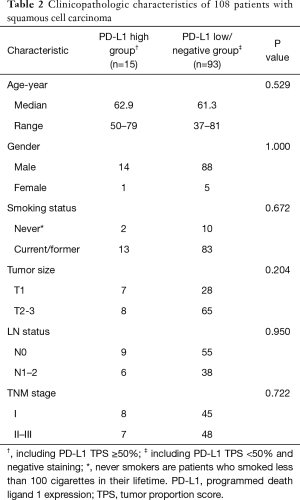
Full table
Full table
A total 319 patients from September 2009 to March 2013 were included for survival analysis. The median follow-up duration of these patients was 23.6 months (range, 0.8–80.0 months). The results showed that patients with PD-L1 high expression (TPS ≥50%) had a significant shorter RFS than patients with PD-L1 low/negative expression (median RFS: 47 vs. 62 months, log-rank P=0.023, Figure 3A). Whereas, there was no significant difference in OS (P=0.679, Figure 3B) between patients with PD-L1 high and low/negative expression. Multivariate analysis showed that PD-L1 status was not an independent risk factor for RFS in the all samples. Therefore, survival analysis was performed in the SCC cohort. Whereas, there were no significant differences in RFS (P=0.393, Figure 3C) and OS (P=0.554, Figure 3D) between patients with PD-L1 high expression and patients with PD-L1 low/negative expression.

Discussion
Over the last decade, with the discovery of various oncogenic driver mutations and development of corresponding molecular agents, the treatment of NSCLC has entered into the era of targeted therapy. Genomic sequencing has revealed different mutation landscapes between Western and East Asian populations. Compared with the highest rate of KRAS mutation in Western population, EGFR mutation in East Asian population ranked the most frequent genetic aberration, which could be effectively inhibited by EGFR-TKIs (10-13).
Although the relationship between oncogenic mutations and immunotherapy efficacy remains unclear, there is a trend that patients suitable for immunotherapy are often lack of known driver mutations (14). In our study, we enrolled 117 LUAD patients who were diagnosed as “pan-negative” by excluding common mutations and translocations, and 104 LUAD patients who harbored one of the six gene aberrations mentioned above. It was interesting that both pan-negative and mutant patients presented low PD-L1 expression, which was significantly different from the high PD-L1 expression rate reported in previous studies (6-8,15). This result indicates that LUAD patients in East Asian population may not benefit from immunotherapy. And this condition is independent of mutation status. However, patients with PD-L1 expression (regardless of TPS) shared some common clinical features, including male, with current/ever smoking history, lack of mutation, solid-predominant subtype, big tumor size and lymph node metastasis, which will be helpful in clinical PD-L1 expression testing. Several studies also reported that smoking history was associated with better immunotherapy response (6,16,17). In our study, we showed smokers are more likely to present high PD-L1 expression. Low proportion of smokers in East Asian LUAD patients may explain the discrepancy of PD-L1 expression between Western and East Asian LUAD populations.
In KEYNOTE-024 study, 500 out of 1,653 patients (30.2%) were diagnosed PD-L1 positive with TPS ≥50% and non-squamous carcinoma is the predominant pathological type (7). This result is totally opposite with our current study. In our study, 34.3% of SCC patients present positive PD-L1 expression, however, only 13.9% of SCC patients could meet the criteria of immunotherapy (with TPS ≥50%). Compared with LUAD, patients with SCC in East Asian are the most suitable population for immunotherapy. Our previous study has shown that SCC in China are characterized by high proportion of never smoker, compared with the reported data of TCGA project from West SCC patients (18-20). This difference may add to the explanation of relatively low PD-L1 expression in East Asian SCC patients. If lung cancer with smoking is defined as a separate disease regardless of pathological type, a significantly different proportion of PD-L1 expression was observed between smoker and never smokers (22.3% vs. 4%, P<0.001).
To our knowledge, this study represents the first comprehensive analysis of PD-L1 expression status with clinical and mutational features by using 22C3 antibody. Previous studies have analyzed the relationship between PD-L1 expression level and clinical parameters, but not with this efficient antibody clone. In addition, we selected surgically resected lung cancer specimens to comprehensively assess the proportion of PD-L1 expression tumor cells, which is superior to biopsy samples often used in the diagnosis of advanced NSCLC. In our study, enrolled patients are almost at early stage. So far, there is no evidence to prove that tumor stage is associated with PD-L1 expression, whether tumor stage is one of the underlying reasons for low PD-L1 expression in East Asian still needed to be explored.
PD-L1 expression was reported to be associated with poor prognosis in NSCLC. In the current study, patients with high PD-L1 expression have an inferior relapse-free survival comparing with low/negative PD-L1 expression group. However, there is no difference between two groups in overall survival. In order to enroll sufficient “pan-negative” LUAD patients into this study, the time span of enrollment varied significantly among three subgroups. The inevitable selection bias may affect the survival analysis.
In conclusion, our study represents a comprehensive PD-L1 expression study with clinicopathological features in East Asian populations, which has a great significance in guiding the clinical screening before immunotherapy. Although the underling molecular mechanism between PD-L1 expression and clinical parameters are still unknown, further next-generation sequencing and mechanism investigation may help to illustrate this issue.
Acknowledgements
Funding: This work was funded by the National Natural Science Foundation of China (81330056, 81572253, 81372525, 81422029, 81572264 and 81601994) and grant from Health and Family Planning Commission of Shanghai Municipality (No. 2013ZYJB0301).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional review board of the Shanghai Cancer Center, Fudan University, Shanghai, China (No. IRB# 090977-1). All patients underwent surgery and provided written informed consent.
References
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-135. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Champiat S, Dercle L, Ammari S, et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin Cancer Res 2017;23:1920-8. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Roach C, Zhang N, Corigliano E, et al. Development of a Companion Diagnostic PD-L1 Immunohistochemistry Assay for Pembrolizumab Therapy in Non-Small-cell Lung Cancer. Appl Immunohistochem Mol Morphol 2016;24:392-7. [Crossref] [PubMed]
- Zhang Y, Sun Y, Pan Y, et al. Frequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosis. Clin Cancer Res 2012;18:1947-53. [Crossref] [PubMed]
- Li H, Pan Y, Li Y, et al. Frequency of well-identified oncogenic driver mutations in lung adenocarcinoma of smokers varies with histological subtypes and graduated smoking dose. Lung Cancer 2013;79:8-13. [Crossref] [PubMed]
- Sun Y, Ren Y, Fang Z, et al. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol 2010;28:4616-20. [Crossref] [PubMed]
- Li C, Fang R, Sun Y, et al. Spectrum of oncogenic driver mutations in lung adenocarcinomas from East Asian never smokers. PLoS One 2011;6:e28204. [Crossref] [PubMed]
- Rangachari D, VanderLaan PA, Shea M, et al. Correlation between Classic Driver Oncogene Mutations in EGFR, ALK, or ROS1 and 22C3-PD-L1 ≥50% Expression in Lung Adenocarcinoma. J Thorac Oncol 2017;12:878-83. [Crossref] [PubMed]
- Uruga H, Bozkurtlar E, Huynh TG, et al. Programmed Cell Death Ligand (PD-L1) Expression in Stage II and III Lung Adenocarcinomas and Nodal Metastases. J Thorac Oncol 2017;12:458-66. [Crossref] [PubMed]
- Sundar R, Soong R, Cho BC, et al. Immunotherapy in the treatment of non-small cell lung cancer. Lung Cancer 2014;85:101-9. [Crossref] [PubMed]
- Lu J, Lee-Gabel L, Nadeau MC, et al. Clinical evaluation of compounds targeting PD-1/PD-L1 pathway for cancer immunotherapy. J Oncol Pharm Pract 2015;21:451-67. [Crossref] [PubMed]
- Huang Y, Wang R, Pan Y, et al. Clinical and genetic features of lung squamous cell cancer in never-smokers. Oncotarget 2016;7:35979-88. [Crossref] [PubMed]
- Pan Y, Wang R, Ye T, et al. Comprehensive analysis of oncogenic mutations in lung squamous cell carcinoma with minor glandular component. Chest 2014;145:473-9. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012;489:519-25. [Crossref] [PubMed]


