Correlation between EGFR mutation status and the incidence of brain metastases in patients with non-small cell lung cancer
Introduction
Brain metastases (BMs) are life-threatening complications of non-small cell lung cancer (NSCLC)£¨accounting for approximately 20–40% of patients (1,2), and are associated with a poor prognosis. The median overall survival (OS) after BMs is only 3 to 7 months despite whole-brain radiation therapy (3-5). Moreover, the development of BMs was concealed under most circumstance which inspired us to seek out a predictive factor of BMs to remind oncologists to screen the BM lesions earlier. Additionally, prophylactic cranial irradiation (PCI), a routine practice for stage I–III small cell lung cancer system treatment (6), may also be used for NSCLC patients. Previous studies have revealed that PCI curtailed the development of BMs in NSCLC patients (7), but it was not routinely delivered due to a lack of improving survival. Therefore, it is critical to identify the high-risk population of BMs.
Recently, several clinical factors, such as younger age, non-squamous cell carcinoma, larger tumor size, lymph node involvement and higher serum tumor markers level (NSE >18 ng/mL, CA125 ≥35 U/mL and CEA ≥23 ng/mL), were observed to be associated with actuarial risk of developing into BMs in NSCLC (8-10). However, risk factors of BMs in molecular level remain to be identified.
Noticeably, epidermal growth factor receptor (EGFR) mutations occur in approximately 20% of lung adenocarcinomas in Western countries (11) and 40–60% in East Asia (12-14). Additionally, the EGFR was routinely detected in clinical practice and widely used as a target for the TKIs in managing patients with BMs. D. Luo et al. (15) showed a similar EGFR mutation frequency (52.9% vs. 46.7%, P=0.644) and a high concordance rate of 93.3% between BMs and the primary NSCLC tumors. Meanwhile, some studies revealed that patients with EGFR mutations were confronted with a higher risk of BMs than those with EGFR wild-type (16-18), whereas others (19,20) argued no distinct occurrence of BMs between them. On that account, we conducted a meta-analysis aiming to evaluate correlation between the EGFR status and incidence of BMs or overall survival calculated from the BMs emerging (BMOS).
Methods
This study was conducted following Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (21) and Meta-analysis of Observational Studies in Epidemiology (MOOSE) (22).
Search strategy
Electronic databases PubMed, Embase, Cochrane Library, CBM, WanFang, CNKI were thoroughly searched to identify relevant trials up to October 2016 without language restriction and were conducted with the following keywords: “brain metastases”, “cerebral metastases”, “neoplasm metastasis”, “central nervous system”, “encephalon”, “epidermal growth factor receptor”, “receptor, epidermal growth factor”, “EGFR”, “EGFR mutation”, “lung neoplasms”, “lung cancer”, “lung carcinoma”, “Pulmonary Neoplasm”, “Pulmonary Cancers”, and “non-small cell lung cancer”. Articles and general reviews of this topic were carefully examined and excluded. Furthermore, we manually reviewed the references of the included studies to screen additional articles.
Selection criteria
Trials meeting the following criteria were included in this study: (I) NSCLC with known mutation status (EGFR mutation or EGFR wild-type); (II) the incidence of BMs could be acquired from EGFR mutation and EGFR wild-type group respectively; (III) BMs were assessed by imaging methods or medical records; (IV) the study design was case control study or cohort study.
Letters, comments, and expert opinions, reviews without original data, and case reports were excluded in this meta-analysis.
Quality assessment
The methodological quality of this meta-analysis was the Newcastle Ottawa Quality Assessment Scale (NOS) (23) and two investigators independently assessed the quality of each study. Any discrepancy was resolved by a third reviewer. This scale with a maximum score nine is composed of eight items and mainly containing patient selection, study comparability and outcome/exposure. Included studies were categorized into high-quality (≥6 score) and low-quality studies (<6 score).
Definition
The BMOS was calculated from the date of occurrence of BMs till the date of the last follow-up or death. Initial BMs were defined as intracranial metastasis appearing at initial diagnosis of NSCLC and subsequent BMs as brain lesions occurring during or after treatment.
Data extraction
Two reviewers extracted data from each trial independently and disagreements were addressed by consensus. The following information was abstracted from each included studies: first author’s name, published date, study type, country, EGFR status, gender, smoking history, histology, Eastern Cooperative Oncology Group (ECOG) performance status, clinical stage, outcomes incorporating the incidence of BMs and BMOS.
Methods of statistics
All data analyses were performed through the Stata/SE 12.0 in this study. The primary endpoints were the incidence of BMs and the secondary endpoints BMOS. Chi-square and I-square tests were used to test the heterogeneity of involved trials. If P>0.1 and I2<50%, the studies were defined as low heterogeneity and fixed effect model was applied, otherwise as high heterogeneity and random effect model was adopted. We also conducted subgroup analyses by study design types (cohort study and case control study), timing of BMs (initial and subsequent) and EGFR mutation types (exon 19 vs. 21). Furthermore, classified analyses were performed on stage IV population and patients with adenocarcinoma, respectively.
Subsequently, we conducted a sensitivity analysis to further evaluate the influence of individual studies on the final conclusion. Incidence of BMs was dichotomous variables and analyzed by pooling odds ratio (OR). We extracted the hazard ratio (HR) and its 95% confidence intervals (CIs) of BMOS from survival curves using the methods described by Tierney et al. (24). Publication bias was assessed via funnel plot and was statistically analyzed using Egger and Begg’s test.
Results
Selection of trials
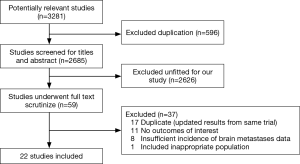
Firstly, 3,281 relevant papers were identified after thoroughly searching the databases. Then, 596 duplicates were excluded. Furthermore, 2,626 papers unfitted design were excluded after reviewing the titles and abstracts. Moreover, full text of 59 papers were intensively scrutinized and 37 were excluded for following reasons: 17 studies for duplication, 11 studies for lacking of outcomes of interest, 8 studies for full-text unavailable, and 1 study for unfitted design. Eventually, 22 studies (16-19,25-42) fulfilling all of the inclusion criteria were eligible for this meta-analysis. A flow chart presented the search results and exclusion reasons (Figure 1).
Study description and quality assessment
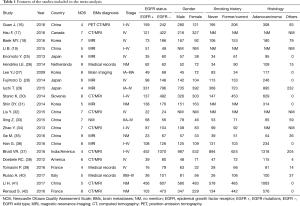
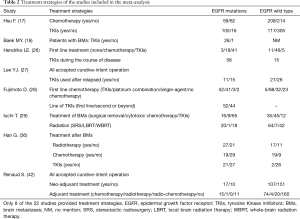
Primary characteristics of these included studies were presented in Table 1. Of all studies, 18 were cohort studies and other 4 case control studies. Additionally, eight of the 22 studies described treatment strategies (Table 2). Among 8,152 patients, 2,664 harbored EGFR mutations.
Full table
Full table
The NOS was used to perform quality assessment on all 22 studies and 14 (17,18,25-28,30,32-36,38,42) were evaluated as high-quality and 8 (16,19,29,31,37,39-41) as low-quality.
Sensitivity analysis
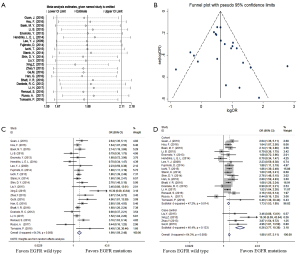
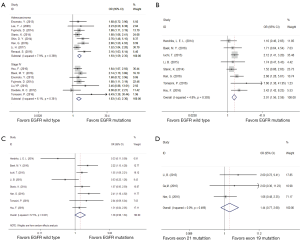
Sensitivity analyses were performed after sequentially removing each eligible study. The pooled OR of BMs were not significant influenced by any single study (Figure 2A), indicating that our results (incidence of BMs) were statistically robust and stable.
Publication bias
The funnel plot, Begg’s test and Egger’s test were used to assess publication bias of the incidence of BMs. The shape of the funnel plots appeared to be generally symmetric (Figure 2B). Both the Begg’s test (P=0.114) and Egger’s test (P=0.253) indicated no publication bias.
Incidence of BMs
All of 22 eligible trials (16-19,25-42) reported incidence of BMs. A random effect model was employed to analyze these studies due to high heterogeneity (P=0.000, I2 =64.3%). Compared with the EGFR wild type group, EGFR mutations group possessed a significantly higher incidence of BMs (OR =1.99; 95% CI, 1.59–2.48; P=0.000) (Figure 2C). Considering the high heterogeneity, analyses stratified by study design (Figure 2D) suggested that association was significant between EGFR mutations and the incidence of BMs in cohort (16-19,25-31,36-42) (OR =1.73; 95%CI, 1.53–1.95; P=0.000) or case control studies (32-35) (OR =6.26; 95% CI, 3.77–10.38; P=0.000). Additionally, the heterogeneity of the involved studies diminished when restricted to adenocarcinoma (25,27,28,30,31,36,41,42) (P=0.369, I2=7.9%) or stage IV (17,18,25,28,32,38,39) (P=0.381, I2=6.1%) (Figure 3A). Patients with EGFR mutations were more susceptible to BMs than wild type cases either in adenocarcinoma patients (OR =1.93; 95% CI, 1.59–2.35; P=0.000) or in advanced (stage IV) NSCLC patients (OR =1.83; 95% CI, 1.43–2.36; P=0.000).
Then several subgroup analyses were conducted to estimate other risks of increasing BMs in NSCLC patients. As respects of timing of BMs (17-19,26,29,30,36,39), compared with EGFR wild type group, EGFR mutations group had a significant higher incidence (OR =2.01; 95% CI, 1.56–2.59; P=0.000) of subsequent BMs (Figure 3B) while only a trend of increasing the incidence of initial BMs (OR =1.38; 95% CI, 0.98–1.94; P=0.066) (Figure 3C). With regard to EGFR mutation type (19,35,36), patients harboring exon 19 mutation suffered a potential higher risk of BMs than those harboring exon 21 mutation (OR =1.44; 95% CI, 0.77–2.68; P=0.252) (Figure 3D).
BMOS
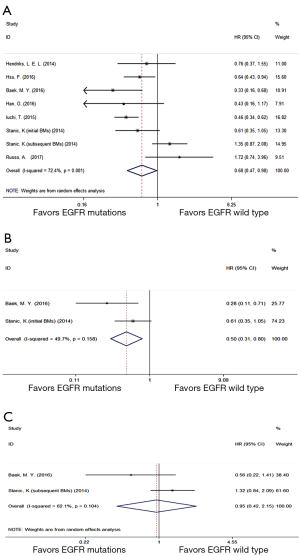
Seven eligible trials (17,18,26,29,30,36,40) compared BMOS between EGFR mutations and wild type group. A random effect model was performed owing to high heterogeneity (P=0.001, I2 =72.4%). Compared with EGFR wild type group, EGFR mutations group displayed a prolonged overall BMOS (HR =0.68; 95% CI, 0.47–0.98; P=0.038) (Figure 4A). Further subgroup analysis (18,30) on BMOS showed that, compared with EGFR wild type group, EGFR mutations group with initial BMs (Figure 4B) gained a longer BMOS (HR =0.50; 95% CI, 0.31–0.80; P=0.004) while those with subsequent BMs (Figure 4C) had an equal BMOS (HR =0.95; 95% CI, 0.42–2.15; P=0.901).
Discussion
To our best knowledge, this meta-analysis of 22 studies incorporating 8,152 participants was the first study to evaluate the risk of BMs in various EGFR status. The results revealed that EGFR mutations were closely associated with a significant higher incidence of BMs (P=0.000). Furthermore, stratified analysis of BMs showed that, compared with EGFR wild type, EGFR mutations group had a significant higher incidence of subsequent BMs and a trend of increasing the incidence of initial BMs. The mechanisms behind it may be as follows: EGFR activated MET through mitogen activated protein kinases (MAPK) to promote BMs in NSCLC (43); moreover, EGFR activated the STAT3 via elevating expression of interleukin-6 (IL-6) in lung cancer which results in the up-regulation of incidence of BMs (44,45). Whereas, the inconsistent conclusions between the initial and subsequent BMs were possibly due to the intervention of EGFR-TKIs. Being highly effective agents for patients harboring EGFR mutations (46), TKIs were more widely used in those cases to prolong OS, which accordingly resulted in more chances of developing BMs in those patients during the course of disease. Additionally, compared with EGFR wild type patients, EGFR mutations had a significantly shorter median Brain-metastasis-free survival (P=0.018) (19), which might also partly account for the higher incidence of BMs of EGFR mutations patients during a given period.
On account of the different clinical characteristics and pathogenesis between exon 19 deletion and 21 point mutations (47-49), we further performed a subgroup analysis to compare the discrepant outcomes between the two kinds of mutations. The results showed that participants with exon 19 deletion displayed a potential trend to promote BMs compared with exon 21 mutation. The mechanisms underlying it might be that patients with EGFR mutations accepted TKIs inhibiting the phosphorylation of EGFR, Akt, and Erk to a greater degree in exon 19 deletion cells than in exon 21 mutation cells (48). Moreover, subgroup analysis on incidence of BMs of patients with lung adenocarcinoma or of patients with stage IV lowered the heterogeneity notably, indicating that both histology and stage might be impact factors for BMs.
Additionally, compared with patients with EGFR wild type, individuals harboring EGFR mutations who suffered from initial BMs had a longer BMOS, but this benefit did not occur in those who suffered from subsequent BMs. The reasons for this finding might be as followings. Compared with EGFR wild type patients, EGFR mutations individuals with initial BMs possessing a longer BMOS might be partly derived from TKIs which yielded a definitely higher response rate and longer time to central nerves system progression (50,51). Furthermore, EGFR mutations individuals with subsequent BMs probably had undergone EGFR-TKIs therapy and developed acquired resistance to some extent when BMs occurred. Accordingly, they could not benefit from EGFR-TKIs anymore, which partly explained the equal BMOS between the EGFR mutations and EGFR wild type group.
However, our study confronted with some limitations: the potential confounding bias of included retrospective studies; the latent mismatched characters, such as age, histology and tumor size, between the EGFR mutations and wild type group; and the impact of various treatment strategies among eligible studies. Thereby, high-quality prospective cohort studies are recommended.
Summarily, patients with EGFR mutations were more susceptible to develop into BMs than those with EGFR wild type, especially during the course of disease. Therefore, careful brain screening and prophylactic interventions possess a potential clinical value for these patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mujoomdar A, Austin JH, Malhotra R, et al. Clinical predictors of metastatic disease to the brain from non-small cell lung carcinoma: primary tumor size, cell type, and lymph node metastases. Radiology 2007;242:882-8. [Crossref] [PubMed]
- Lombardi G, Di Stefano AL, Farina P, et al. Systemic treatments for brain metastases from breast cancer, non-small cell lung cancer, melanoma and renal cell carcinoma: an overview of the literature. Cancer Treat Rev 2014;40:951-9. [Crossref] [PubMed]
- Eichler AF, Chung E, Kodack DP, et al. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol 2011;8:344-56. [PubMed]
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419-25. [Crossref] [PubMed]
- Mehta MP, Rodrigus P, Terhaard CH, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol 2003;21:2529-36. [Crossref] [PubMed]
- Woolf DK, Slotman BJ, Faivre-Finn C. The Current Role of Radiotherapy in the Treatment of Small Cell Lung Cancer. Clin Oncol (R Coll Radiol) 2016;28:712-9. [Crossref] [PubMed]
- Gore EM, Bae K, Wong SJ, et al. Phase III comparison of prophylactic cranial irradiation versus observation in patients with locally advanced non-small-cell lung cancer: primary analysis of radiation therapy oncology group study RTOG 0214. J Clin Oncol 2011;29:272-8. [Crossref] [PubMed]
- Ma X, Zhu H, Guo H, et al. Risk factors of brain metastasis during the course of EGFR-TKIs therapy for patients with EGFR-mutated advanced lung adenocarcinoma. Oncotarget 2016;7:81906-17. [PubMed]
- Ji Z, Bi N, Wang J, et al. Risk factors for brain metastases in locally advanced non-small cell lung cancer with definitive chest radiation. Int J Radiat Oncol Biol Phys 2014;89:330-7. [Crossref] [PubMed]
- Hubbs JL, Boyd JA, Hollis D, et al. Factors associated with the development of brain metastases: analysis of 975 patients with early stage nonsmall cell lung cancer. Cancer 2010;116:5038-46. [Crossref] [PubMed]
- Dogan S, Shen R, Ang DC, et al. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin Cancer Res 2012;18:6169-77. [Crossref] [PubMed]
- Sun Y, Ren Y, Fang Z, et al. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol 2010;28:4616-20. [Crossref] [PubMed]
- Wu JY, Yu CJ, Chang YC, et al. Effectiveness of tyrosine kinase inhibitors on "uncommon" epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res 2011;17:3812-21. [Crossref] [PubMed]
- Wu YL, Lee JS, Thongprasert S, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol 2013;14:777-86. [Crossref] [PubMed]
- Luo D, Ye X, Hu Z, et al. EGFR mutation status and its impact on survival of Chinese non-small cell lung cancer patients with brain metastases. Tumour Biol 2014;35:2437-44. [Crossref] [PubMed]
- Guan J, Chen M, Xiao N, et al. EGFR mutations are associated with higher incidence of distant metastases and smaller tumor size in patients with non-small-cell lung cancer based on PET/CT scan. Med Oncol 2016;33:1. [Crossref] [PubMed]
- Hsu F, De Caluwe A, Anderson D, et al. EGFR mutation status on brain metastases from non-small cell lung cancer. Lung Cancer 2016;96:101-7. [Crossref] [PubMed]
- Baek MY, Ahn HK, Park KR, et al. Epidermal growth factor receptor mutation and pattern of brain metastasis in patients with non-small cell lung cancer. Korean J Intern Med 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Li B, Sun SZ, Yang M, et al. The correlation between EGFR mutation status and the risk of brain metastasis in patients with lung adenocarcinoma. J Neurooncol 2015;124:79-85. [Crossref] [PubMed]
- Eichler AF, Kahle KT, Wang DL, et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol 2010;12:1193-9. [Crossref] [PubMed]
- Panic N, Leoncini E, de Belvis G, et al. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One 2013;8:e83138. [Crossref] [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [Crossref] [PubMed]
- Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Accessed 12nd January 2017. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [Crossref] [PubMed]
- Enomoto Y, Takada K, Hagiwara E, et al. Distinct features of distant metastasis and lymph node stage in lung adenocarcinoma patients with epidermal growth factor receptor gene mutations. Respir Investig 2013;51:153-7. [Crossref] [PubMed]
- Hendriks LE, Smit EF, Vosse BA, et al. EGFR mutated non-small cell lung cancer patients: more prone to development of bone and brain metastases? Lung Cancer 2014;84:86-91. [Crossref] [PubMed]
- Lee YJ, Park IK, Park MS, et al. Activating mutations within the EGFR kinase domain: a molecular predictor of disease-free survival in resected pulmonary adenocarcinoma. J Cancer Res Clin Oncol 2009;135:1647-54. [Crossref] [PubMed]
- Fujimoto D, Ueda H, Shimizu R, et al. Features and prognostic impact of distant metastasis in patients with stage IV lung adenocarcinoma harboring EGFR mutations: importance of bone metastasis. Clin Exp Metastasis 2014;31:543-51. [Crossref] [PubMed]
- Iuchi T, Shingyoji M, Itakura M, et al. Frequency of brain metastases in non-small-cell lung cancer, and their association with epidermal growth factor receptor mutations. Int J Clin Oncol 2015;20:674-9. [Crossref] [PubMed]
- Stanic K, Zwitter M, Hitij NT, et al. Brain metastases in lung adenocarcinoma: impact of EGFR mutation status on incidence and survival. Radiol Oncol 2014;48:173-83. [Crossref] [PubMed]
- Shin DY, Na II, Kim CH, et al. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol 2014;9:195-9. [Crossref] [PubMed]
- Liu Y. Risk factors for brain metastases in advanced non-small cell lung cancer. Guangxi medical University, 2015. Available online: http://cdmd.cnki.com.cn/Article/CDMD-10598-1015330948.htm
- Xing Z, Wang H, Qun H, et al. Risk factors and survival analysis of locally advanced non-small cell lung cancer with brain metastasis. J Clin Med 2015;19:24-7.
- Zhao Y. A prediction model for brain metastasis in non-small cell lung cancer. Peking Union Medical College, 2013. Available online: http://cdmd.cnki.com.cn/article/cdmd-10023-1013312334.htm
- Ge M, Zhou X, Zhan Q, et al. Non-small cell lung cancer with brain metastasis harbored more mutant EGFR gene. Chin J Cancer Biother 2016;23:387-91.
- Han G, Bi J, Tan W, et al. A retrospective analysis in patients with EGFR-mutant lung adenocarcinoma: is EGFR mutation associated with a higher incidence of brain metastasis? Oncotarget 2016;7:56998-7010. [Crossref] [PubMed]
- Bhatt VR, D’Souza SP, Smith LM, et al. Epidermal Growth Factor Receptor Mutational Status and Brain Metastases in Non–Small-Cell Lung Cancer. J Glob Oncol 2016;3:208-17. [Crossref] [PubMed]
- Doebele RC, Lu X, Sumey C, et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer 2012;118:4502-11. [Crossref] [PubMed]
- Tomasini P, Serdjebi C, Khobta N, et al. EGFR and KRAS Mutations Predict the Incidence and Outcome of Brain Metastases in Non-Small Cell Lung Cancer. Int J Mol Sci 2016.17. [PubMed]
- Russo A, Franchina T, Ricciardi GR, et al. Influence of EGFR mutational status on metastatic behavior in non squamous non small cell lung cancer. Oncotarget 2017;8:8717-25. [PubMed]
- Li H, Cao J, Zhang X, et al. Correlation between status of epidermal growth factor receptor mutation and distant metastases of lung adenocarcinoma upon initial diagnosis based on 1063 patients in China. Clin Exp Metastasis 2017;34:63-71. [Crossref] [PubMed]
- Renaud S, Seitlinger J, Falcoz PE, et al. Specific KRAS amino acid substitutions and EGFR mutations predict site-specific recurrence and metastasis following non-small-cell lung cancer surgery. Br J Cancer 2016;115:346-53. [Crossref] [PubMed]
- Breindel JL, Haskins JW, Cowell EP, et al. EGF receptor activates MET through MAPK to enhance non-small cell lung carcinoma invasion and brain metastasis. Cancer Res 2013;73:5053-65. [Crossref] [PubMed]
- Singh M, Garg N, Venugopal C, et al. STAT3 pathway regulates lung-derived brain metastasis initiating cell capacity through miR-21 activation. Oncotarget 2015;6:27461-77. [Crossref] [PubMed]
- Gao SP, Mark KG, Leslie K, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest 2007;117:3846-56. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Zhu JQ, Zhong WZ, Zhang GC, et al. Better survival with EGFR exon 19 than exon 21 mutations in gefitinib-treated non-small cell lung cancer patients is due to differential inhibition of downstream signals. Cancer Lett 2008;265:307-17. [Crossref] [PubMed]
- Rossi S, D'Argento E, Basso M, et al. Different EGFR Gene Mutations in Exon 18, 19 and 21 as Prognostic and Predictive Markers in NSCLC: A Single Institution Analysis. Mol Diagn Ther 2016;20:55-63. [Crossref] [PubMed]
- Li M, Zhang Q, Liu L, et al. The different clinical significance of EGFR mutations in exon 19 and 21 in non-small cell lung cancer patients of China. Neoplasma 2011;58:74-81. [Crossref] [PubMed]
- Luo S, Chen L, Chen X, et al. Evaluation on efficacy and safety of tyrosine kinase inhibitors plus radiotherapy in NSCLC patients with brain metastases. Oncotarget 2015;6:16725-34. [Crossref] [PubMed]
- Jiang T, Min W, Li Y, et al. Radiotherapy plus EGFR TKIs in non-small cell lung cancer patients with brain metastases: an update meta-analysis. Cancer Med 2016;5:1055-65. [Crossref] [PubMed]