Noninvasive ventilation during acute respiratory distress syndrome in patients with cancer—what really matters
Noninvasive ventilation (NIV) is used to provide ventilator support with the aim of avoiding the complications of invasive mechanical ventilation. These include direct lesions of the patients’ pharyngeal, laryngeal and tracheal structures, and, more importantly, nosocomial infections. Of the latter, ventilator-associated pneumonia (VAP) is the most relevant, but the need for sedation to tolerate the invasive airway and the more invasive monitoring may result in increased infectious complications outside the lungs as well (1,2). Groups of patients who benefit most from NIV include exacerbation of chronic obstructive pulmonary disease (COPD), acute cardiogenic pulmonary edema, and patients with immunosuppression (1-3).
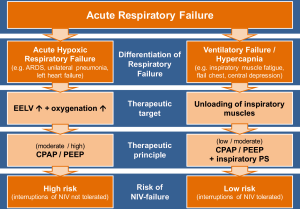
Acute respiratory failure is one of the major problems leading to admission to the intensive care unit (ICU). Although this term is often used for a variety of clinical situations, it can be useful to differentiate two major groups of underlying causes. In a landmark paper published in 1982, Roussos and Macklem (4) suggested distinguishing between gas-exchange failure of the lungs, which mainly leads to hypoxemia, and failure of the muscular “pump”, which usually causes hypercapnic acidosis. This pathophysiological classification of the underlying cause of respiratory failure cannot always be applied in mixed forms, but consideration of it should have a major impact on ventilator treatment strategy.
The primary target in ventilatory pump failure is to support the patients’ respiratory muscular effort whenever necessary. This can be achieved by a low positive end-expiratory pressure (PEEP) or continuous positive airway pressure (CPAP) and moderate to high inspiratory pressure support. If complete decompensation of the ventilator muscle pump can be avoided, this support can usually be interrupted, which comprises a therapeutic domain for NIV.
On the other hand, in patients with impaired gas-exchanging function, the primary target is to restore the reduced end-expiratory lung volume (EELV) without further damaging the inflamed and vulnerable lung by mechanical stress associated with high transpulmonary driving pressures caused by both patient and ventilator (5,6). This can be mainly achieved by ventilator support that uses moderate to high PEEP or CPAP, which may restore impaired EELV and lung compliance and thereby often normalizes work of breathing and ventilator drive. As denoted by the term CPAP, the pressure should be applied continuously and without interruption, which could otherwise result in immediate re-collapse of recruited alveoli (7). A moderate to high CPAP with no or only minor inspiratory pressure support can be generally applied by a non-invasive airway interface such as a face mask or helmet, but NIV is by nature an intermittent form of ventilator support, which has to be paused on the long term due to patient discomfort, removal of secretions, etc. The helmet can be used for longer at a stretch (8) and is more capable of attaining higher and reliable PEEP/CPAP application (9).
The primary cause of impaired EELV and arterial oxygenation is acute respiratory distress syndrome (ARDS), caused by an acute inflammatory lung injury and non-cardiogenic pulmonary edema (10). In a recent international prospective observational study (LUNG-SAFE trial) (11), over 10% of 29,144 patients admitted to the ICU fulfilled ARDS criteria. The authors summarized that ARDS is still often unrecognized, undertreated and associated with high hospital mortality rate between 35% (mild ARDS) and 46% (severe ARDS).
The LUNG-SAFE trial showed that NIV is used in about 15% of patients with ARDS independent of ARDS severity (11). NIV failure rates were up to 45% in most severe ARDS patients and patients with a PaO2/FiO2 <150 mmHg that were treated with NIV showed an increased ICU mortality compared to invasive mechanical ventilation from the outset (12).
ARDS in patients with cancer
In a recent issue of the Journal of Critical Care, Neuschwander and colleagues (13) analyzed the use and outcome of NIV during ARDS in the special group of patients with cancer. The objectives of the study were to describe the outcome of patients with different types of malignancies and ARDS treated with NIV and to evaluate factors associated with NIV failure. Therefore, they retrospectively analyzed individual patient data from six previously published studies (prospective und retrospective) of patients with cancer who required ICU admission between 1990–2011 in 14 university centers in France and Belgium. ARDS was retrospectively defined according to the current Berlin Definition criteria (10). NIV was defined as a procedure involving a face or nasal mask for more than one hour. The use of NIV for preoxygenation before endotracheal intubation and the use in the treatment of obstructive sleep apnea syndrome were excluded. Finally, NIV failure was defined as the need of invasive mechanical ventilation during the ICU stay.
They conclude that NIV was frequently used during ARDS in patients with cancer regardless of the severity with peak use at the beginning of the 2000s. An ARDS related to pulmonary infection, a severe ARDS and other additional organ dysfunction like acute kidney injury or shock were to be associated with NIV failure. The NIV failure itself, the severe form of ARDS and associated organ failure where associated with hospital mortality.
NIV in patients with cancer—is everything clear now?
As the authors mentioned themselves, there are several aspects in the study which need to be discussed as potential limitations possibly leading to wrong conclusions.
First of all, the patient characteristics according to the underlying cancer are not well distributed. There is a high rate of hematologic malignancies in the two groups (80% in the group of patients never received NIV vs. 94% in the group of patients who received NIV) and only a small rate of solid tumors (20% vs. 6%). The type of solid tumors is not mentioned although it may have an impact if it is a primary lung tumor or a tumor with pulmonary metastases. Furthermore, the stage of cancer is not specified in the text and there is no information given about patients’ refusal of invasive mechanical ventilation or other limitations of medical treatment.
The second important point is NIV itself. The data was collected within 21 years [1990–2011]. In this period, the importance of NIV changed and there were also multiple changes in the management of patient with ARDS in the ICU. Given this limitation, the lack of information in the study about the ventilator settings like the level of PEEP, pressure support, tidal volume or length of NIV-sessions is particularly relevant. As known from other studies, even when respiratory failure is recognized as an ARDS, mechanical ventilation is often inadequate (e.g., inadequately low PEEP, larger tidal volume). In their study, NIV was used in all ARDS subgroups with no significant difference between the groups and it tended to be lowest in mild ARDS group though a contradiction between the text and the related Figure 2 confuse the issue. The low use of NIV in mild ARDS seems surprising, but may be due to underdiagnosis of ARDS in line with recent findings (11).
Furthermore, delayed intubation could not be assessed in the study. This is especially important because this could have a notable effect on the higher mortality for the patients with NIV failure. The association between delayed intubation and mortality has been shown in before (14). This may in part explain why patients with NIV failure in the study had a significantly higher rate of shock, acute kidney failure and a higher SOFAC (SOFA score without respiratory parameters) compared to the patients with NIV success. It also remains unclear which aspects finally lead to NIV failure. Was it the increasing respiratory failure under NIV therapy or another cause like a reduced mental status (GCS ≤8) or a hemodynamically unstable patient as a result of additional organ dysfunction?
As argued above, it is crucial to clearly define the major problem leading to mechanical ventilation—a ventilatory failure or an oxygenation failure (see Figure 1). Patients with an oxygenation failure leading to hypoxemia need lung recruitment with CPAP to hinder alveolar recollapse. So NIV as a mostly discontinuous treatment may be less effective in this group of patients. On the other hand, patients with ventilatory failure, which often manifests with hypercapnia due to central depression, fatigue or a mechanical defect of the chest wall and respiratory muscles, should benefit from NIV. Given the huge changes that have taken place in oncological management over the past decades, it would seem more important to focus on such distinctions in choosing ventilation strategies and less on tailoring them to underlying co-morbidities.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med 2001;344:481-7. [Crossref] [PubMed]
- Ram FS, Picot J, Lightowler J, et al. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2004.CD004104. [PubMed]
- Vital FM, Ladeira MT, Atallah AN. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev 2013.CD005351. [PubMed]
- Roussos C, Macklem PT. The respiratory muscles. N Engl J Med 1982;307:786-97. [Crossref] [PubMed]
- Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013;369:2126-36. [Crossref] [PubMed]
- Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015;372:747-55. [Crossref] [PubMed]
- Neumann P, Berglund JE, Andersson LG, et al. Effects of inverse ratio ventilation and positive end-expiratory pressure in oleic acid-induced lung injury. Am J Respir Crit Care Med 2000;161:1537-45. [Crossref] [PubMed]
- Principi T, Pantanetti S, Catani F, et al. Noninvasive continuous positive airway pressure delivered by helmet in hematological malignancy patients with hypoxemic acute respiratory failure. Intensive Care Med 2004;30:147-50. [Crossref] [PubMed]
- Patel BK, Wolfe KS, Pohlman AS, et al. Effect of Noninvasive Ventilation Delivered by Helmet vs Face Mask on the Rate of Endotracheal Intubation in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2016;315:2435-41. [Crossref] [PubMed]
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Noninvasive Ventilation of Patients with Acute Respiratory Distress Syndrome. Insights from the LUNG SAFE Study. Am J Respir Crit Care Med 2017;195:67-77. [Crossref] [PubMed]
- Neuschwander A, Lemiale V, Darmon M, et al. Noninvasive ventilation during acute respiratory distress syndrome in patients with cancer: Trends in use and outcome. J Crit Care 2017;38:295-9. [Crossref] [PubMed]
- Carrillo A, Gonzalez-Diaz G, Ferrer M, et al. Non-invasive ventilation in community-acquired pneumonia and severe acute respiratory failure. Intensive Care Med 2012;38:458-66. [Crossref] [PubMed]


